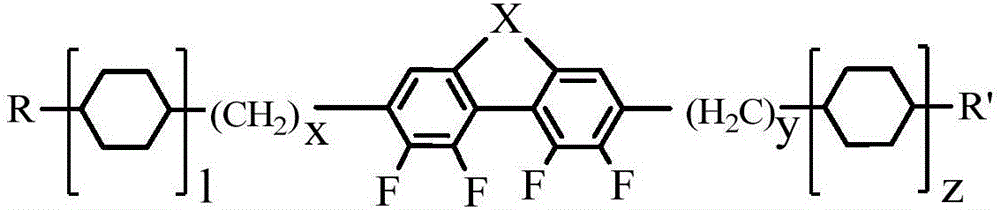
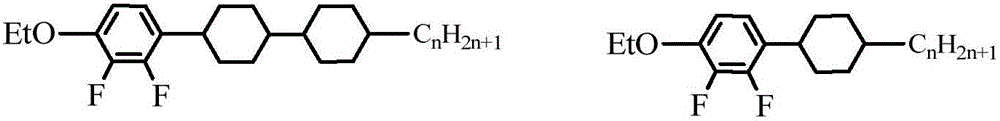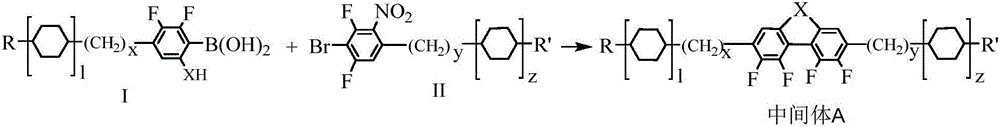Laterally tetrafluoro-substituted dibenzoheterocyclic compound and preparation method thereof
A technology of heterocyclic compounds and compounds, applied in the field of materials, can solve the problems of small absolute value of dielectric anisotropy, difficult to improve the response speed, etc., and achieve large negative dielectric anisotropy, high product yield and purity, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 3-(but-3-enyl)-7-propyl-1,2,8,9-tetrafluorobibenzo[b,d]furan of the following structure
[0044]
[0045] 1. Suzuki coupling reaction
[0046] Under nitrogen protection at a flow rate of 0.6 mL / min, 7.10 g (21 mmol) of 4-bromo-3,5-difluoro-2-nitrophenylpropionaldehyde ethylene glycol acetal, 4.32 g (20 mmol) of 2,3 -Difluoro-6-hydroxy-4-n-propylphenylboronic acid, 6.44g (20mmol) tetra-n-butylammonium bromide, 7.18g (52mmol) potassium carbonate, 30mL N,N-dimethylformamide, 10mL distilled water Add it into a three-necked flask, raise the temperature to 65°C, and after the solid is completely dissolved, add 0.73g (0.63mmol) tetrakis(triphenyl)phosphine palladium, react at 65°C for 2 hours, add 130mL of distilled water, extract with ethyl acetate, organic Phase was washed with distilled water to neutrality, using silica gel as stationary phase and ethyl acetate as eluent column chromatography to obtain 5.91g of intermediate A-1 with the following structur...
Embodiment 2
[0063] Preparation of 3-butyl-7-propyl-1,2,8,9-tetrafluorobibenzo[b,d]furan with the following structural formula
[0064]
[0065] In Example 1, the 4-bromo-3,5-difluoro-2-nitrophenylpropionaldehyde ethylene glycol acetal used equimolar 3-bromo-2,4-difluoro-6-n-butyl Nitrobenzene replacement, according to the method of Example 1, through Suzuki coupling reaction, reduction reaction, fluorination reaction, to obtain 3-butyl-1,2,8,9-tetrafluoro-7-propyl bibenzo[b , d] furan, the total yield was 42%.
[0066] The NMR data of the obtained 3-butyl-1,2,8,9-tetrafluoro-7-propylbibenzo[b,d]furan are as follows:
[0067] 13 C-NMR (CDCl 3 as solvent, internal standard is TMS, 75MHz, ppm): 153.8, 146.0, 143.6, 143.4, 142.3, 142.0, 124.1, 123.5, 113.7, 107.9, 107.2, 106.9, 33.4, 31.4, 28.9, 24.1, 22.3, 14.1, 13.7 .
[0068] 1 H-NMR (CDCl 3 As a solvent, the internal standard is TMS, 300MHz, ppm): 6.98 (2H, s), 2.6 (4H, t), 1.59-1.65 (4H, m), 1.31 (2H, t), 0.90 (6H, t).
Embodiment 3
[0070] Preparation of 3-(but-3-enyl)-7-(trans-4-n-propylcyclohexyl)-1,2,8,9-tetrafluorobibenzo[b,d]furan of the following structure
[0071]
[0072] In Example 1, 2,3-difluoro-6-hydroxyl-4-n-propylphenylboronic acid was used with equimolar 2,3-difluoro-6-hydroxyl-4-(trans-4-n-propyl Cyclohexyl)-phenylboronic acid replacement, other steps are the same as in Example 1 to obtain 3-(but-3-enyl)-7-(trans-4-n-propylcyclohexyl)-1,2,8,9- Tetrafluorobibenzo[b,d]furan, the total yield is 43%.
[0073] The NMR data of the obtained 3-(but-3-enyl)-7-(trans-4-n-propylcyclohexyl)-1,2,8,9-tetrafluorobibenzo[b,d]furan are as follows:
[0074] 13 C-NMR (CDCl 3 as solvent, internal standard is TMS, 75MHz, ppm): 153.8, 146.0, 143.6, 143.4, 143.2, 143.0, 138.3, 124.9, 124.5, 116.4, 113.8, 113.0, 107.9, 107.5, 34.4, 31.4, 28.6, 24.1, 13.7 .
[0075] 1 H-NMR (CDCl 3 as solvent, internal standard is TMS, 300MHz, ppm): 6.98(1H, s), 6.96(1H, s), 5.82(1H, m), 5.02-5.07(2H, m), 2.56-2.62(3H, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com