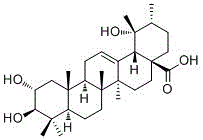
Medicine composition for treating neurodermatitis
A composition and neurological technology, applied in the direction of drug combination, neurological diseases, skin diseases, etc., can solve problems such as no treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the pharmaceutical composition for the treatment of neurodermatitis and preparation method thereof
[0033] The composition and parts by weight of the raw material drug of the pharmaceutical composition for treating neurodermatitis are: Potentinic acid 28g, prolactin 1285g, polyalgae 843g;
[0034] Preparation:
[0035] (1) According to the proportion of raw materials, take Potentinic acid, prolactin, polyalgae, mix well, use ethanol with a concentration of 39% by weight as a solvent, and extract by warm soaking at 36.5°C. The number of extractions is 13 times, and the extraction time for each For 1 hour, the amount of solvent each time is 22.5 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A recovered ethanol, concentrated to a relative density of 1.06, filtered, and the drug solution passed through FU-02 The macroporous adsorption resin column is firstly eluted with water, and then the FU-0...
Embodiment 2
[0038] Embodiment 2: the pharmaceutical composition for the treatment of neurodermatitis and preparation method thereof
[0039]The composition and parts by weight of the raw material drug of the pharmaceutical composition for treating neurodermatitis are: 26g of pallinic acid, 1290g of prolactin and 840g of polyalgae;
[0040] Preparation:
[0041] (1) According to the proportion of raw materials, take Potentinic acid, prolactin, polyalgae, mix well, use ethanol with a concentration of 39% by weight as a solvent, and extract by warm soaking at 36.5°C. The number of extractions is 13 times, and the extraction time for each For 1 hour, the amount of solvent each time is 22.5 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A recovered ethanol, concentrated to a relative density of 1.06, filtered, and the drug solution passed through FU-02 The macroporous adsorption resin column is firstly eluted with water, and then...
Embodiment 3
[0044] Embodiment 3: the pharmaceutical composition for the treatment of neurodermatitis and preparation method thereof
[0045] The composition and parts by weight of the raw material drug of the pharmaceutical composition for treating neurodermatitis are: 30g of pallinic acid, 1280g of prolactin and 846g of polyalgae;
[0046] Preparation:
[0047] (1) According to the proportion of raw materials, take Potentinic acid, prolactin, polyalgae, mix well, use ethanol with a concentration of 39% by weight as a solvent, and extract by warm soaking at 36.5°C. The number of extractions is 13 times, and the extraction time for each For 1 hour, the amount of solvent each time is 22.5 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A recovered ethanol, concentrated to a relative density of 1.06, filtered, and the drug solution passed through FU-02 The macroporous adsorption resin column is firstly eluted with water, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com