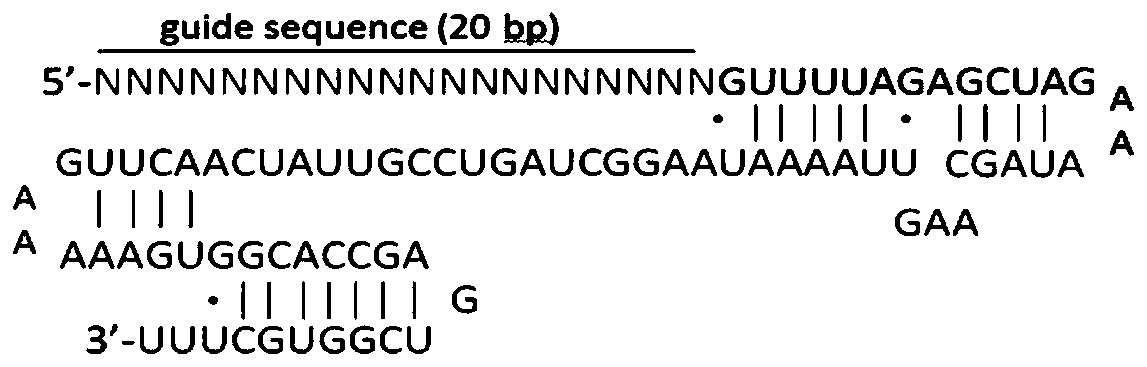A kind of plasmid and preparation for directional clearance of hbvcccDNA in hepatocytes
A preparation and auxiliary lipid technology, which is applied in the field of biomacromolecular pharmaceutical preparations, can solve the problems of lack of safe and effective preparations for in vivo application, and achieve the effects of reducing the expression level of antigens, overcoming instability in vivo, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Design, synthesis and eukaryotic expression vector construction of sgRNA sequence
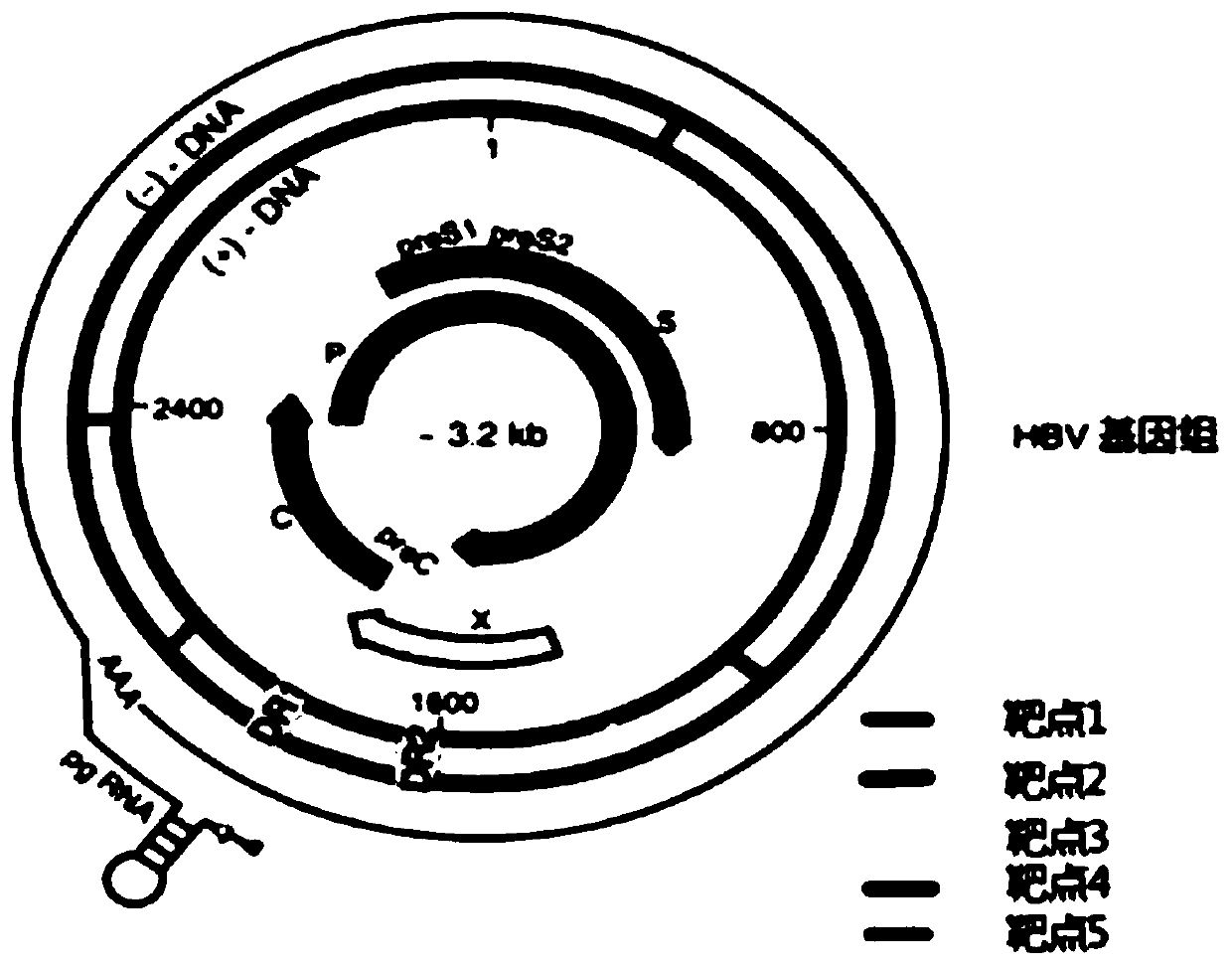
[0079] We chose to target different subtypes of HBV genomes: adw (Genebank ID: AF100309.1), adw2 (Genebank ID: X02763), adr (Genebank ID: AF411411), ayw (Genebank ID: V01460.1) as CRISPR / Cas The targeting sequence of the system is used as the targeting sequence of the CRISPR / Cas system, and multiple sgRNAs targeting the conserved regions of the viral genome were designed according to the sgRNA design principles. Compare the obtained sgRNA with the human genome sequence, exclude the sgRNA with high homology, and finally find five new sgRNA targets and target sequences (as shown in Table 1 & as shown in Table 1). figure 1 shown).
[0080] Table 1
[0081]
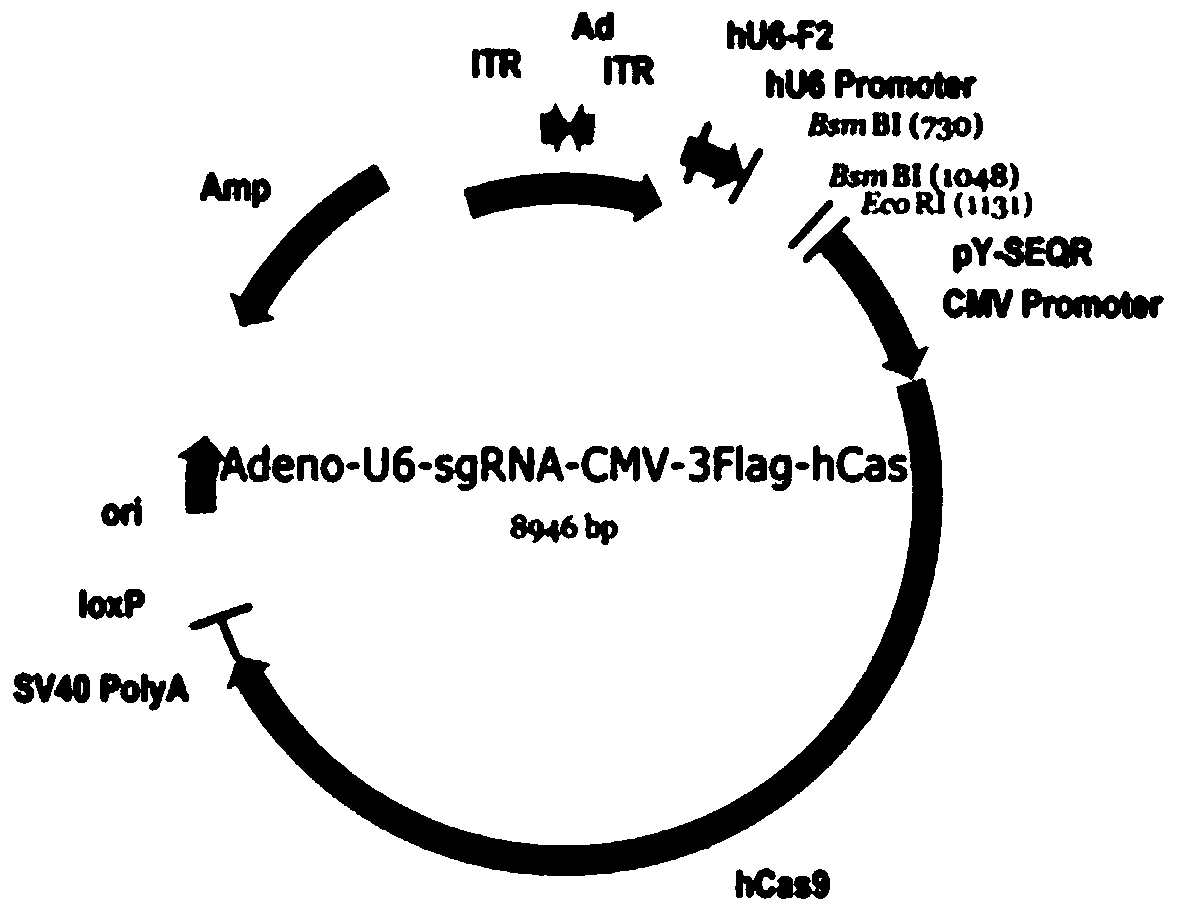
[0082]According to the selected target sequence, we first synthesized the corresponding sgRNA insert sequence: GN 19, and the GN 19 sequence was annealed and cloned into the linearized pAdeno-U6-sgRNA-CMV-3Flag-hCas9 vect...
Embodiment 2
[0090] Five kinds of CRISPR / Cas9 expression plasmids (sg1, sg2, sg3, sg4, sg5) constructed in Example 1 were co-transfected into SMMC-7721 cells with the pHBV1.3 plasmid, and the virus surface antigen, e antigen, Changes in the cccDNA of hepatitis B virus, and based on this, the effects of five CRISPR / Cas9 systems on inhibiting HBV replication were evaluated.
[0091] (1) Cell transfection After mixing pHBV 1.3 and CRISPR / Cas9 plasmids, SMMC-7721 cells were transfected with lipofectamine 2000, and pHBV 1.3 was mixed with pGl3 plasmids as a control group. Cultured for 48h after transfection, culture medium and cells were collected for subsequent assay experiments.
[0092] (2) Determination of hepatitis B surface antigen and e antigen The above cells were centrifuged, and the cell supernatant was collected. The hepatitis B surface antigen and e antigen in the supernatant were measured with the hepatitis B virus surface antigen diagnostic kit and the hepatitis B virus e antigen...
Embodiment 3
[0100] The preparation of embodiment 3 cationic lipid nucleic acid drug preparation
[0101] Cationic lipids are prepared using DOTAP as an example, and nucleic acid drugs are prepared using CRISPR / Cas9 as an example (sg1, sg2, sg3, sg4, sg5).
[0102] The pH-responsive lipid used in this example is lipid CA, which is a prior art. The structural formula of the lipid CA is as shown in formula II, specifically:
[0103]
[0104] Those skilled in the art can use the method reported in the document "Controlling HBV Replication in Vivo by Intravenous Administration of Triggered PEGylated siRNA-Nanoparticles" to prepare and obtain lipid CA.
[0105] Such as Figure 4 As shown, the lipid CA reacts with PEG whose terminal group is an aldehyde group to obtain PEGylated lipid CA. The structural formula of the PEGylated lipid CA is shown in formula III, specifically:
[0106]
[0107] (1) Preparation of blank liposomes: Take a certain amount of DOTAP / DOPE / CA lipid stock solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com