Non-viral gene vector for gene transfer as well as preparation method and use thereof
A gene vector, non-viral technology, applied to the non-viral gene vector for gene transfer and the fields of its preparation and use, can solve the problems of unsatisfactory transfection efficiency, reduced cytotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
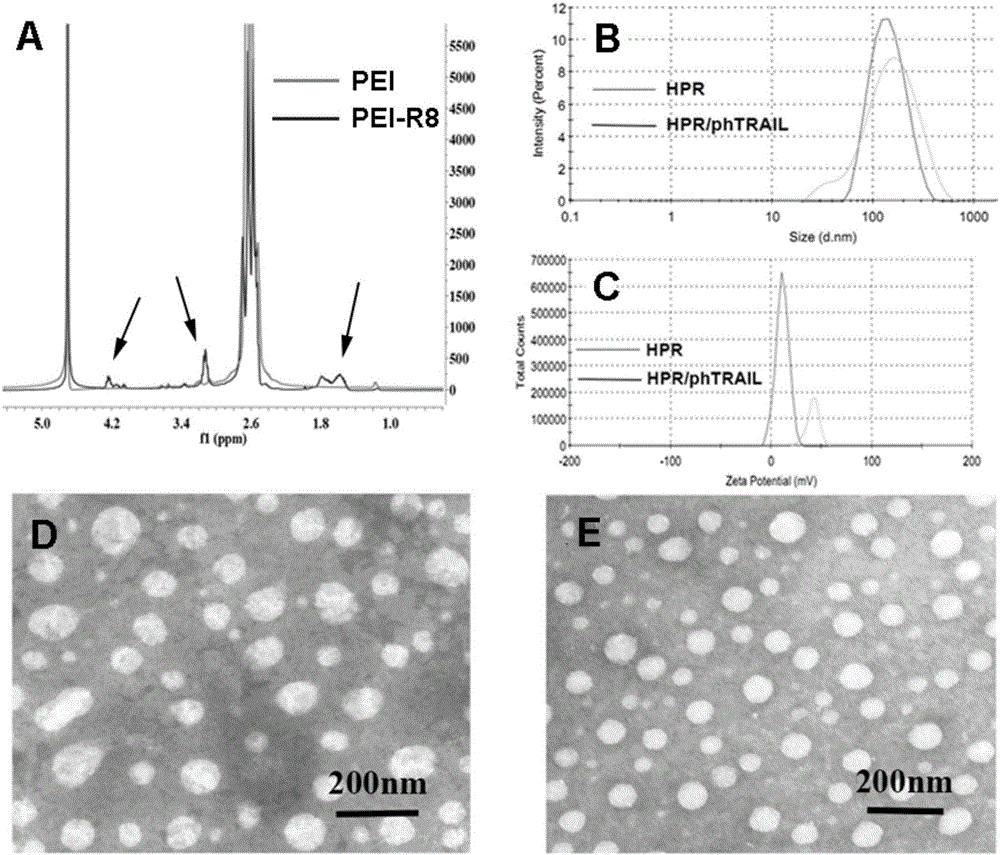
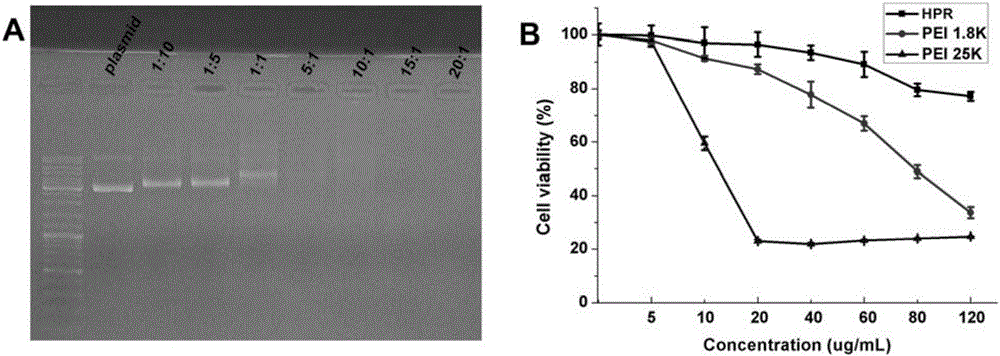
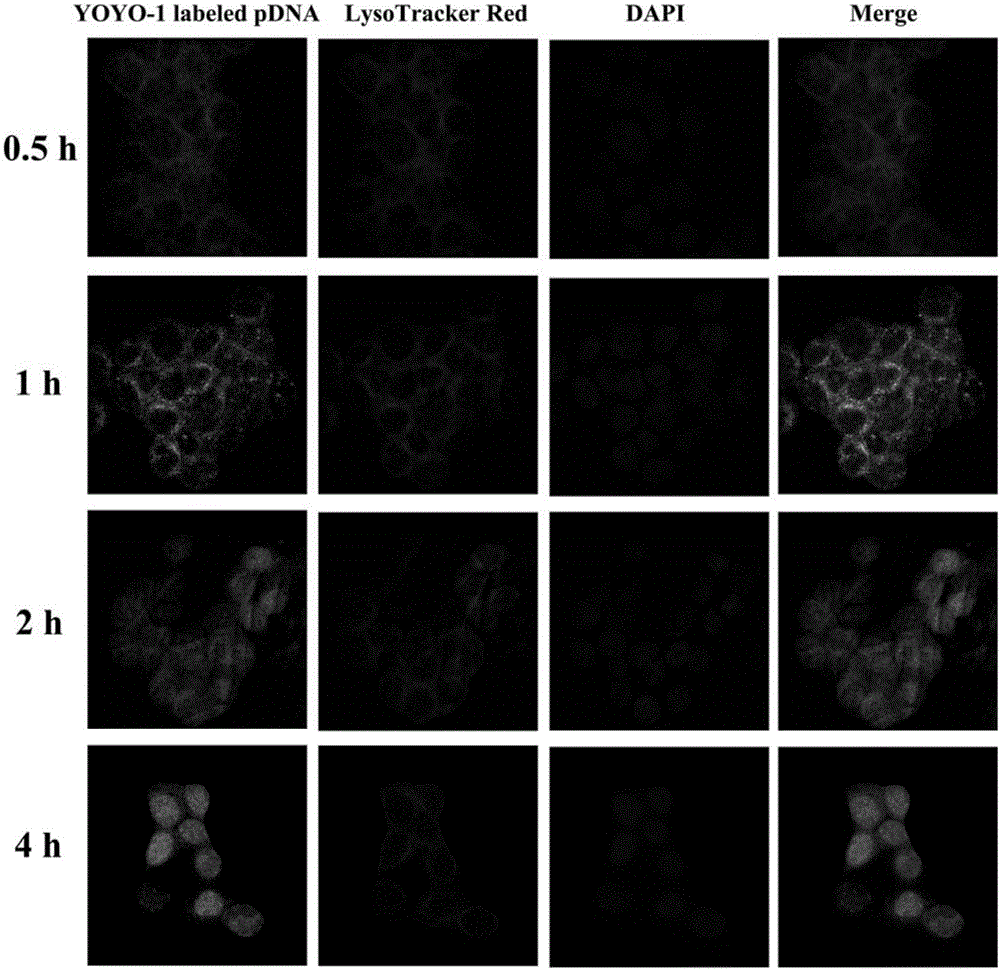
[0028] Embodiment 1 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0029] 1. Preparation method
[0030] (1) Preparation of PEI-R8
[0031] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at -20°C for future use.
[0032] (2) Preparation of HPR
[0033] Dissolve 50 mg of heparin sodium in 100 mL of MES buffe...
Embodiment 2
[0040] Embodiment 2 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0041] 1. Preparation method
[0042] (1) Preparation of PEI-R8
[0043] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at minus 20 degrees for future use.
[0044] (2) Preparation of HPR
Embodiment 3
[0046] Embodiment 3 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0047] 1. Preparation method
[0048] (1) Preparation of PEI-R8
[0049] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at minus 20 degrees for future use.
[0050] (2) Preparation of HPR
[0051] Dissolve 50 mg of heparin sodium in 100 mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com