Transient Transfection Process of Drosophila Cells
A transient transfection, Drosophila technology, applied in the field of bioengineering, can solve the problems of limiting the application of TGE technology, and the protein expression yield of insect cells cannot achieve the expected results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
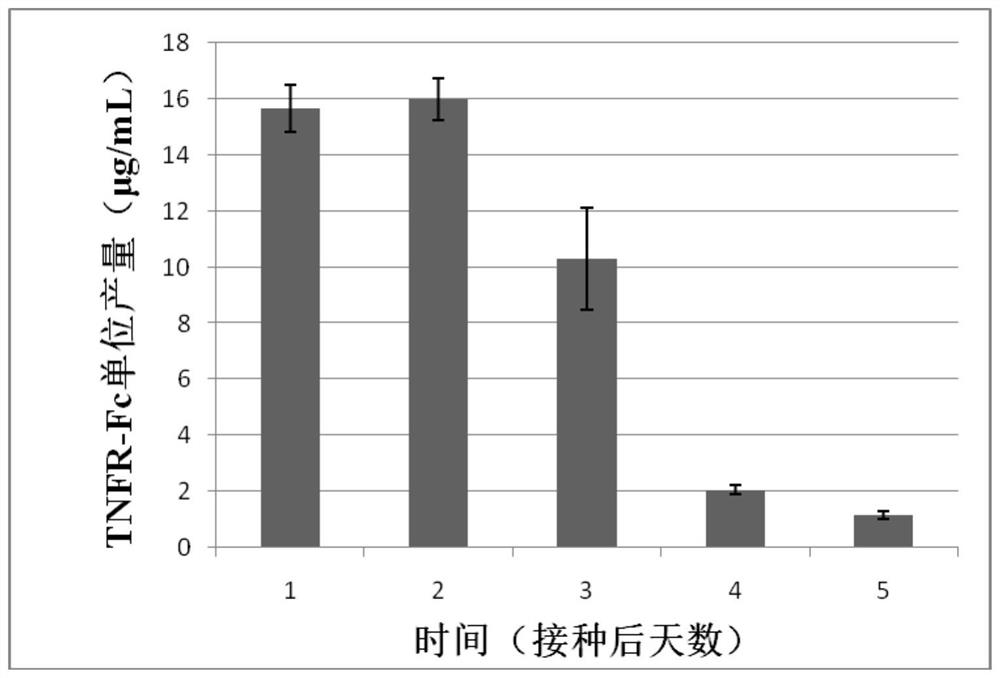
[0043] Example 1 provides a method for expressing the secreted protein TNFR-Fc using the technique of transient transfection of Drosophila cells of the present invention. The parameters used in this example have been optimized. It can be understood that in other examples, Not limited to this.
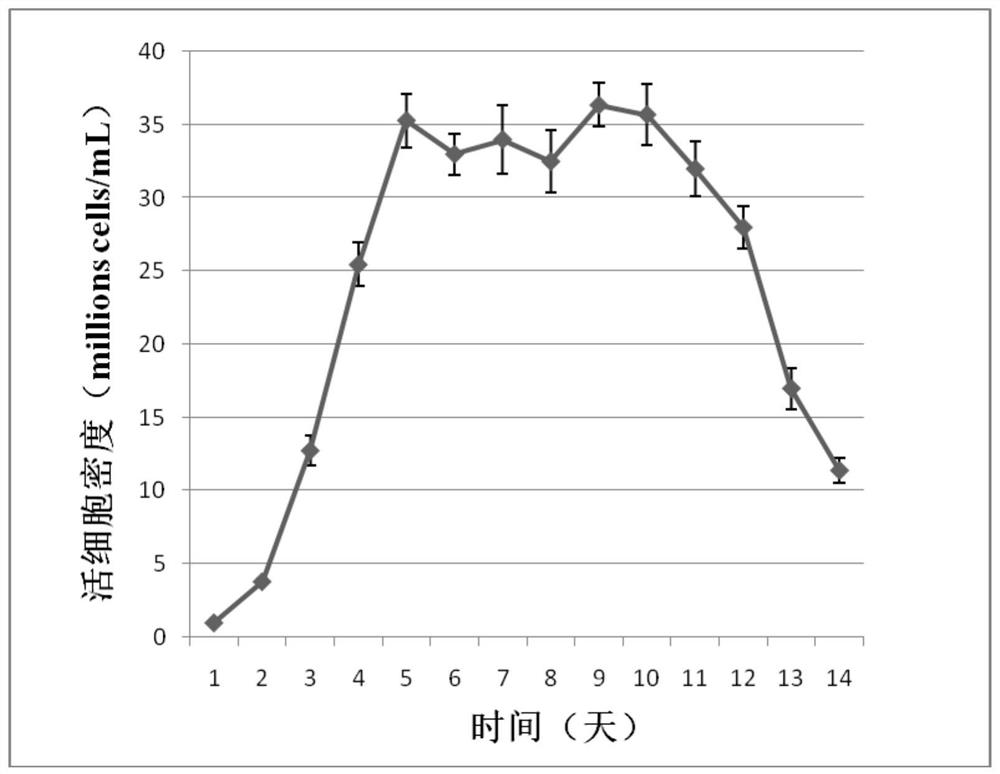
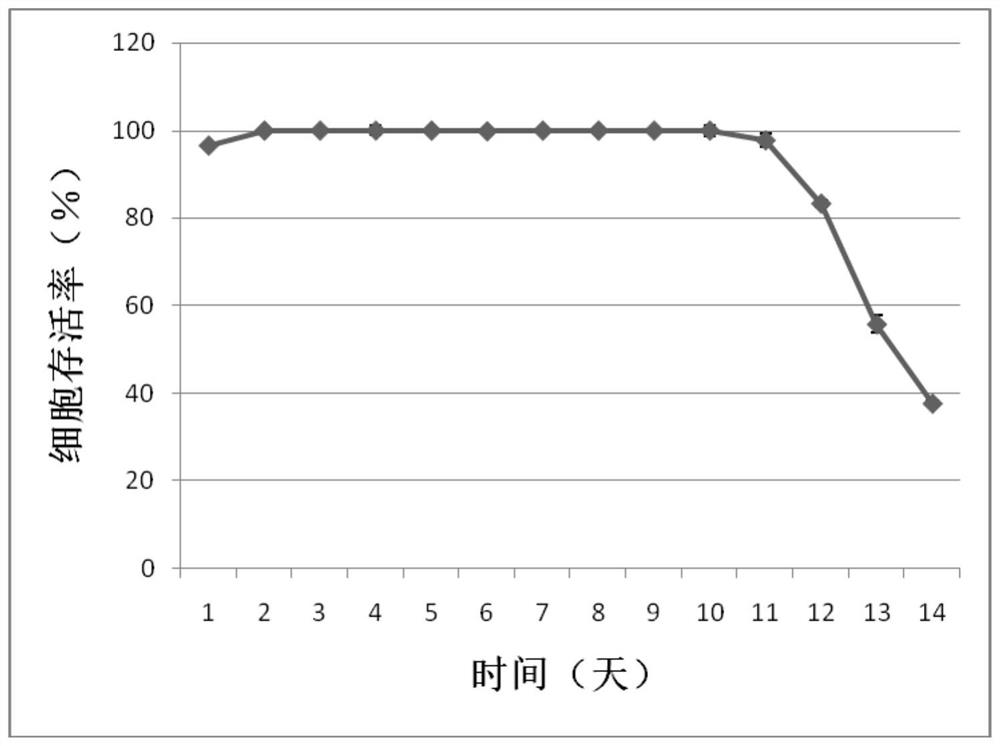
[0044] Dilute Drosophila S2 cells at 1×10 6 The density of Drosophila cells / mL was subcultured into Sf900II SFM medium, and shake culture was carried out at 28°C and 280rpm → cultured for 2 days to logarithmic production phase, centrifuged and resuspended to a density of 15×10 6 Drosophila cells / mL→according to 0.6μg / 10 6 pDNA of a Drosophila cell and 2.0 μg / 10 6 PEI of Drosophila cells was added to the transfection system, and the cells were diluted to 5×10 after 1 hour. 6 Drosophila cells / mL → after 5 days of culture, the protein expression level was detected by ELISA.
[0045] The process can be scaled up from 10mL to 300mL and beyond. The specific process of expressing TNFR-Fc ...
Embodiment 2
[0059] Example 1 provides a method for expressing the intracellular protein EGFP using the technique of transient transfection of Drosophila cells. The parameters used in this example have been optimized, and it is understandable that in other examples, it is not limited thereto.
[0060] Dilute Drosophila S2 cells at 1×10 6 The density of Drosophila cells / mL was subcultured into Sf900II SFM medium, and shake culture was carried out at 28°C and 280rpm → cultured for 3 days to logarithmic production phase, centrifuged and resuspended to a density of 15×10 6 Drosophila cells / mL→according to 0.6μg / 10 6 pDNA of a Drosophila cell and 2.0 μg / 10 6 PEI of Drosophila cells was added to the transfection system, and the cells were diluted to 5×10 after 1 hour. 6 Drosophila cells / mL → after 3 days of culture, detect the percentage of EGFP-positive cells by GUAVAEasyCyte flow cytometer, and perform fluorescence quantification by TECAN SaphirelI fluorescence photometer.
[0061] The proc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com