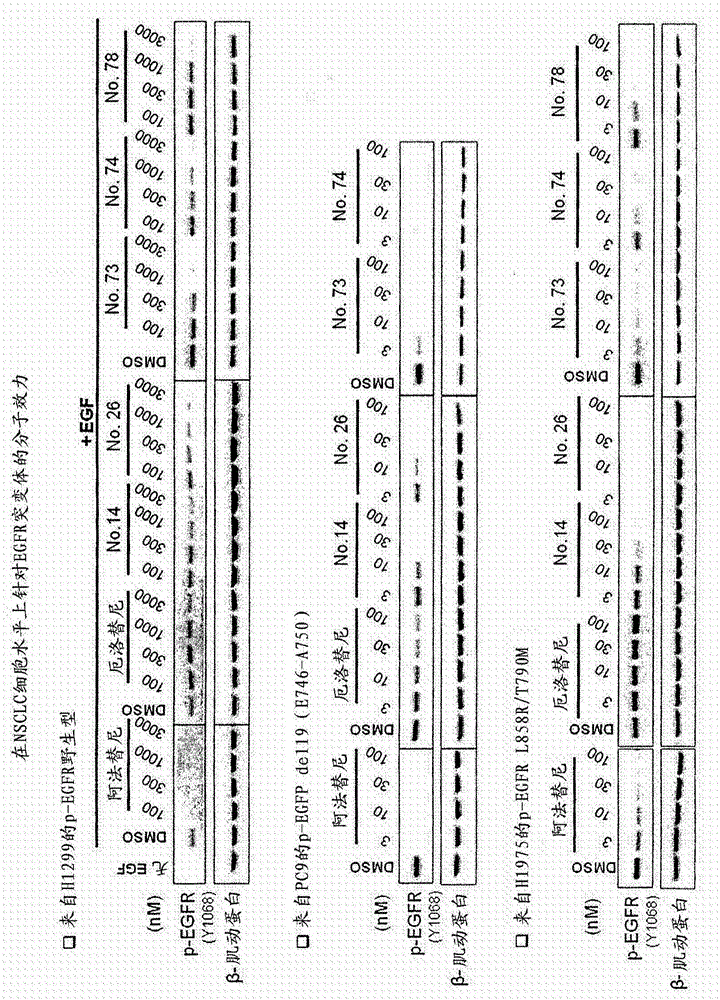
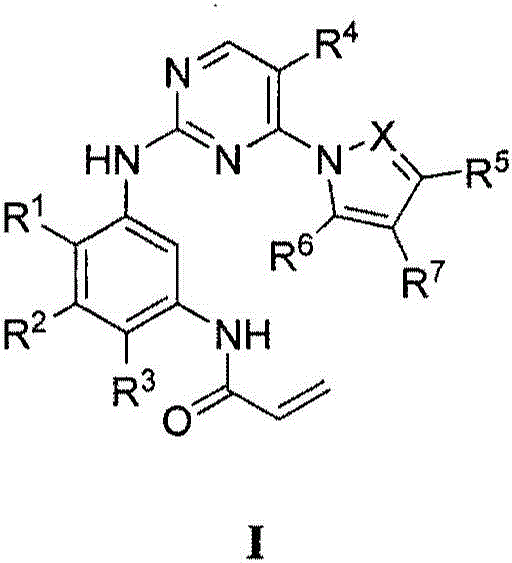
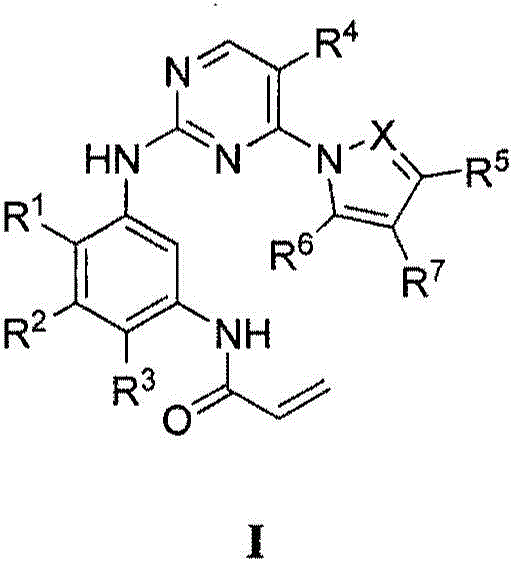
Compounds and compositions for modulating EGFR mutant kinase activities
A technology of compounds and medicinal salts, applied in the field of compounds and compositions for regulating the kinase activity of EGFR mutants, capable of solving problems such as precise effects, small pleiotropic defects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0607] Compound 1: N-(3-(4-(4-(azetidin-1-ylmethyl)-3-methyl-1H-pyrazol-1-yl)pyrimidine-2- (amino)-4-methoxyphenyl)acrylamide
[0608]
[0609] step 1:
[0610] To a solution of Intermediate 1 (35.0 mg, 0.10 mmol), diisopropylethylamine (DIPEA, 50 μL, 0.30 mmol) in dimethylacetamide (DMAA, 2 mL) was added 18.5 mg of the azacycline at room temperature Butane hydrochloride (0.20 mmol). After stirring for 20 minutes, 62.8 mg of sodium triacetoxyborohydride (NaBH(OAc) 3 , 0.30 mmol), and the resulting mixture was stirred at room temperature for 16 hours. The solvent was evaporated in vacuo and the mixture was purified by column chromatography (0 to 10% MeOH in DCM) to afford 4-(4-(azetidin-1-ylmethyl)-3-methyl as a red solid -1H-pyrazol-1-yl)-N-(2-methoxy-5-nitrophenyl)pyrimidin-2-amine (32.0 mg, 82%); MS (ESI) m / z 396.2 [M +H] + .
[0611] Step 2:
[0612] To a solution of the above nitro compound (56.0 mg, 0.14 mmol) in 3 mL of a mixture of ethanol and water (5...
Embodiment 2
[0616] Compound 2: N-(3-(4-(4-((3-hydroxyazetidin-1-yl)methyl)-3-methyl-1H-pyrazol-1-yl) Pyrimidin-2-ylamino)-4-methoxyphenyl)acrylamide
[0617] The title compound was prepared as described in Example 1 using Intermediate 1 and azetidin-3-ol hydrochloride; MS (ESI) m / z 436.2 [M+H] + .
Embodiment 3
[0619] Compound 3: N-(3-(4-(4-(azetidin-1-ylmethyl)-3-methyl-1H-pyrazol-1-yl)pyrimidine-2- Amino)phenyl)acrylamide
[0620] The title compound was prepared as described in Example 1 using Intermediate 2 and azetidine hydrochloride; MS (ESI) m / z 390.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com