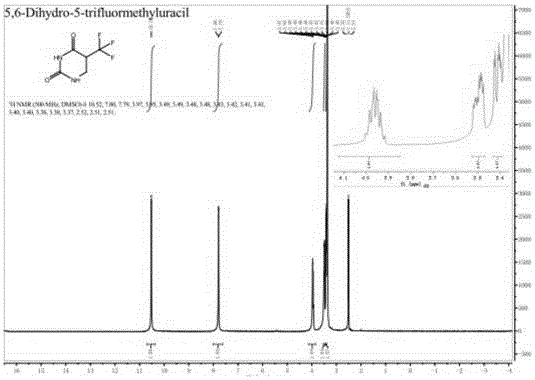
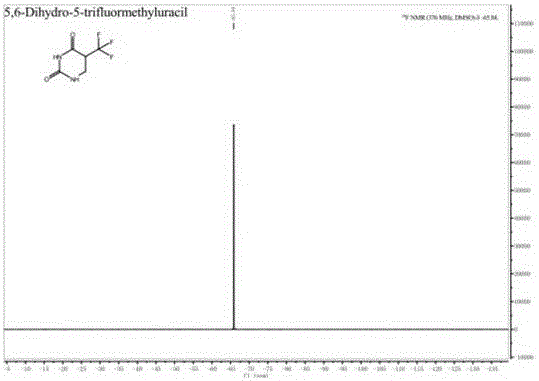
Preparation method of 5-trifluoromethyl-5,6-dihydrouracil
A technology of dihydrouracil and trifluoromethyl, applied in the field of preparation of 5-trifluoromethyl-5,6-dihydrouracil, which can solve problems such as narrow commercial access, complicated process, and potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
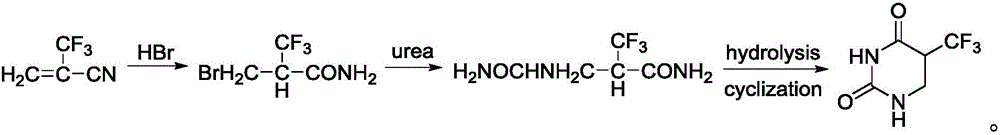
[0034] The invention provides a preparation method of 5-trifluoromethyl-5,6-dihydrouracil, comprising the following steps:
[0035] a) adding 2-trifluoromethacrylic acid and hydrogen bromide in a solvent to obtain 3-bromo-2-trifluoromethacrylic acid;
[0036] b) mixing the 3-bromo-2-trifluoromethylacrylic acid obtained in step a) with ammonia water, and performing an ammoniation reaction to obtain 3-amino-2-trifluoromethylpropionic acid;
[0037] c) Condensing the 3-amino-2-trifluoromethylpropionic acid obtained in step b) and cyanate in an acid solution to obtain 5-trifluoromethyl-5,6-dihydrouracil.
[0038] In the present invention, firstly, 2-trifluoromethacrylic acid and hydrogen bromide are subjected to an addition reaction in a solvent to obtain 3-bromo-2-trifluoromethacrylic acid. In the present invention, the 2-trifluoromethacrylic acid has a structure shown in formula (I):
[0039]
[0040] In the present invention, there is no special limitation on the sources o...
Embodiment 1
[0064] (1) Add 56.0g (0.40mol) of 2-trifluoromethacrylic acid and 120mL of chloroform into a 250mL four-necked reaction flask, mix well under mechanical stirring, then cool the reaction flask to 0°C, Slowly feed 38.9 g (0.48 mol) of hydrogen bromide gas under low pressure. After the gas is passed, continue the reaction for 1 h, then stop cooling, and naturally return to room temperature to continue the reaction for 12 h. After recovering the solvent by rotary evaporation, 3-bromo-2-tri Fluoromethylpropionic acid 84.0g; the yield is 95%, and the purity is 98.5%.
[0065] (2) Under the protection of nitrogen, slowly pour 88.4g (0.4mol) of 3-bromo-2-trifluoromethylpropionic acid into the reactor, then add 300mL (2.16mol) of ammonia water, close the reaction system, and lower the temperature of the reactor to Raised to 80°C, and kept at 80°C for 4 hours. After the reaction, the material was released, cooled and crystallized, filtered, and dried to obtain 60.8g of 3-amino-2-trifluo...
Embodiment 2
[0070] (1) Add 56.0g (0.40mol) of 2-trifluoromethacrylic acid and 120mL of chloroform into a 250mL four-necked reaction flask, mix well under mechanical stirring, then cool the reaction flask to 0°C, Slowly feed 38.9 g (0.48 mol) of hydrogen bromide gas under low pressure. After the gas is passed, continue the reaction for 1 h, then stop cooling, and naturally return to room temperature to continue the reaction for 12 h. After recovering the solvent by rotary evaporation, 3-bromo-2-tri Fluoromethylpropionic acid 84.0g; the yield is 95%, and the purity is 98.5%.
[0071] (2) Under the protection of nitrogen, slowly pour 88.4g (0.4mol) of 3-bromo-2-trifluoromethylpropionic acid into the reactor, then add 300mL (2.16mol) of ammonia water, close the reaction system, and lower the temperature of the reactor to Raised to 80°C, and kept at 80°C for 2 hours. After the reaction, the material was released, cooled and crystallized, filtered, and dried to obtain 50.9g of 3-amino-2-trifluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com