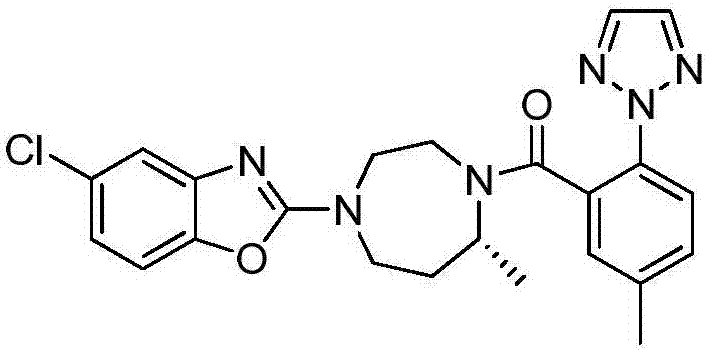
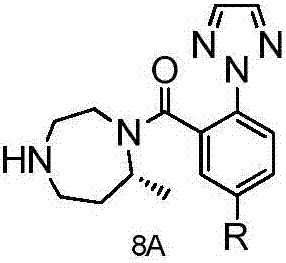
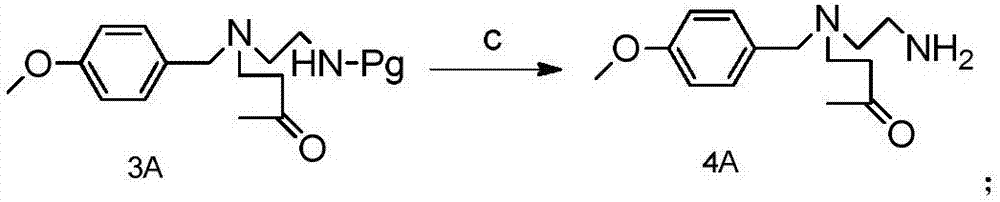
Method for preparing Suvorexantintermediate and analogue thereof
A technology for analogs and intermediates, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of inconvenient process operation, large environmental pollution, waste of raw materials, etc., achieve simple and feasible process operation, simple and easy operation process, and reduce environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] (a) Synthesis of Compound 2:
[0152]
[0153] In a 5L reaction flask, add absolute ethanol (1.7L), then add p-methoxybenzaldehyde (200g, 1.47mol), anhydrous sodium sulfate (104g, 0.734mol), glacial acetic acid (6g, 0.1mol), continue to stir for 10min after the addition is complete, then add dropwise the mixed solution of Boc-ethylenediamine (282g, 1.763mol) and 300mL ethanol, after the dropwise completion, heat up to 62°C for 2h, then cool down to room temperature, add Sodium triacetoxyborohydride (343g, 1.62mol), stirred for 1h, monitored by HPLC
[0154] After the reaction is complete, add 400 mL of water and stir for 15 minutes, then concentrate the reaction solution under reduced pressure until no solution drops out, add 2 L of water to the residue, stir well, adjust the pH value of the solution to 3 with 2N hydrochloric acid, and extract impurities with diethyl ether. The pH value of the aqueous phase was adjusted to 8 with sodium bicarbonate, and the product ...
Embodiment 2
[0188] (a) Synthesis of Compound 2:
[0189]
[0190] In a 5L reaction flask, add ethanol (1.7L), then add 200g of p-methoxybenzaldehyde, 104g of anhydrous sodium sulfate, and 6g of glacial acetic acid while stirring. The mixture of diamine and 300mL of ethanol was added, and the temperature was raised to 70°C for 2 hours, then cooled to room temperature, 343g of sodium triacetoxyborohydride was added, stirred for 1 hour, and monitored by HPLC.
[0191] After the reaction is complete, add 400 mL of water and stir for 15 minutes, then concentrate the reaction solution under reduced pressure until no solution drops out, add 2 L of water to the residue, and stir evenly; adjust the pH value of the solution to 3 with 2N hydrochloric acid, and extract impurities with MTBE; The pH value was adjusted to 8 with sodium bicarbonate, the product was extracted with EA, the EA phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain...
Embodiment 3
[0218] (a) Synthesis of Compound 2:
[0219]
[0220] In a 10L reaction flask, add ethanol (3.4L), then add 400g of p-methoxybenzaldehyde, 208g of anhydrous sodium sulfate, and 12g of glacial acetic acid while stirring. The mixture of ethylenediamine and 600mL of ethanol was added, and the temperature was raised to 70°C for 2 hours, then cooled to room temperature, 686g of sodium triacetoxyborohydride was added, stirred for 1 hour, and monitored by HPLC.
[0221] After the reaction is complete, add 800 mL of water, stir for 15 min, then concentrate the reaction solution under reduced pressure until no solvent drips out, add 4 L of water to the residue, stir evenly, adjust the pH value of the solution to 3 with 2N hydrochloric acid, extract impurities with MTBE, water The pH value of the phase was adjusted to 8 with sodium bicarbonate, and the product was extracted with EA. The EA phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com