Oral solid preparation of regorafenib and preparation method of oral solid preparation
A solid preparation, regorafenib technology, applied in the field of medicine, can solve the problems of less regorafenib preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The following examples are used to further illustrate the present invention, to further understand a kind of regorafenib oral solid preparation and its preparation method, but the present invention is not limited thereto.
[0023] The tablets compressed in the following examples and comparative examples, unless otherwise specified, were all compressed with the same type of tablet press, and the hardness was controlled within the range of 60-100N.
[0024] 1. Test
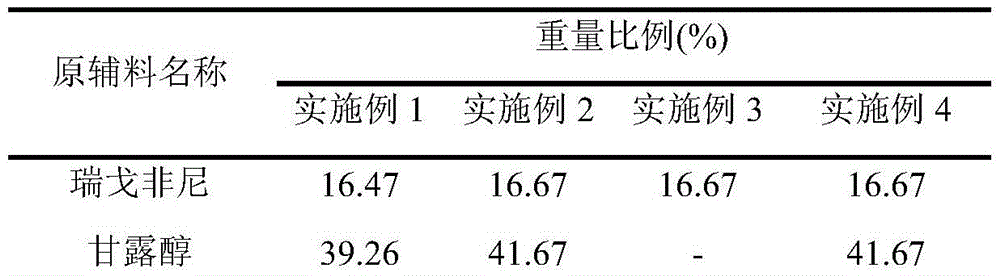
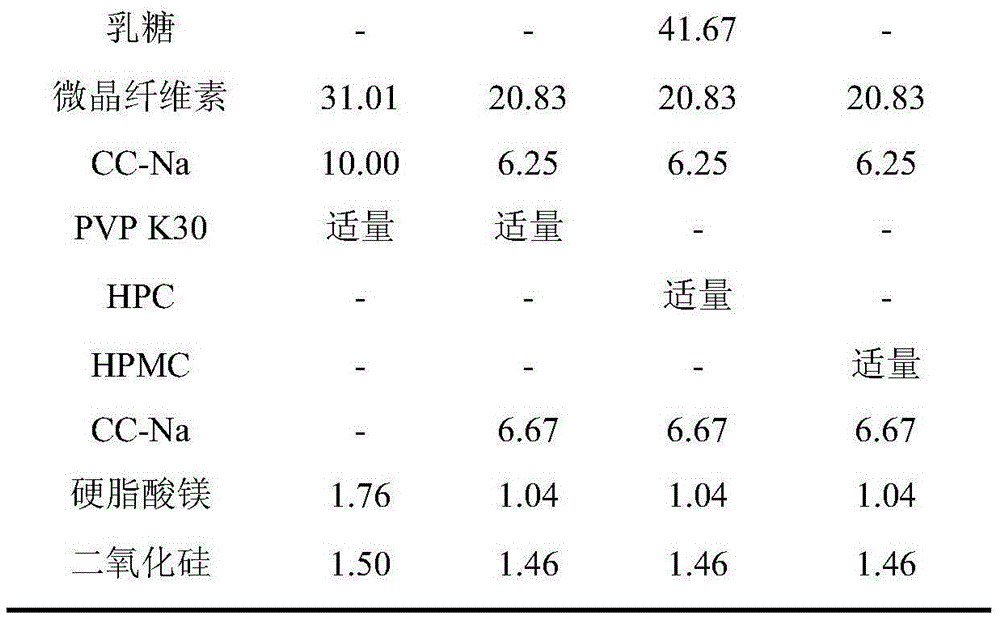
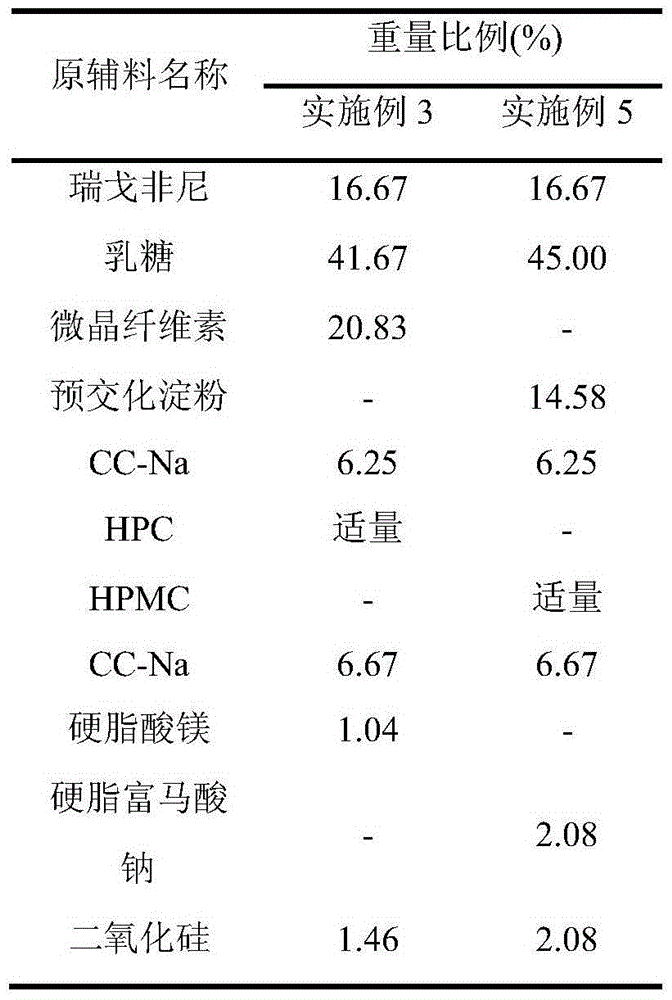
[0025] Table 1
[0026]
[0027]
[0028] Preparation Process:
[0029] (1) Grinding regorafenib to a particle size of less than 30 μm, mixing the pulverized raw material with mannitol / lactose, microcrystalline cellulose, and croscarmellose sodium (CC-Na);
[0030] (2) granulate the mixture obtained in step (1) with 2% povidone (PVP K30) / hydroxypropyl cellulose (HPC) / hypromellose (HPMC) aqueous solution;
[0031] (3) mix the mixture obtained in step (2) with partially croscarmellose sodium, silicon d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com