Organic compound with long afterglow effect as well as preparation method and application of organic compound
An organic compound, long afterglow technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of harsh synthesis conditions, doping inorganic rare earth materials, and complexity, and achieve convenient preparation and simple reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of PhCz: First, 1 times the equivalent of carbazole, 1 to 1.5 times the equivalent of halogenated benzene, such as bromobenzene, fluorobenzene, etc., 0.44 times the equivalent of Cu (electrolytically refined copper), 4.04 times the equivalent of K 2 CO 3 Put it into a reaction vessel, use nitrobenzene as the reaction solvent, and react at 120-160°C for 12-48 hours under the protection of nitrogen. The reaction solution is distilled under reduced pressure to obtain the crude product of 9-phenylcarbazole, and the pure product is obtained by column chromatography. The rate is 85-95%.
[0026]
[0027] Its hydrogen spectrum 1 H NMR (400MHz, CDCl 3 ), δ (TMS, ppm): 8.21 (d, 2H), 7.66 (m, 4H), 7.52 (m, 5H), 7.34 (m, 2H).
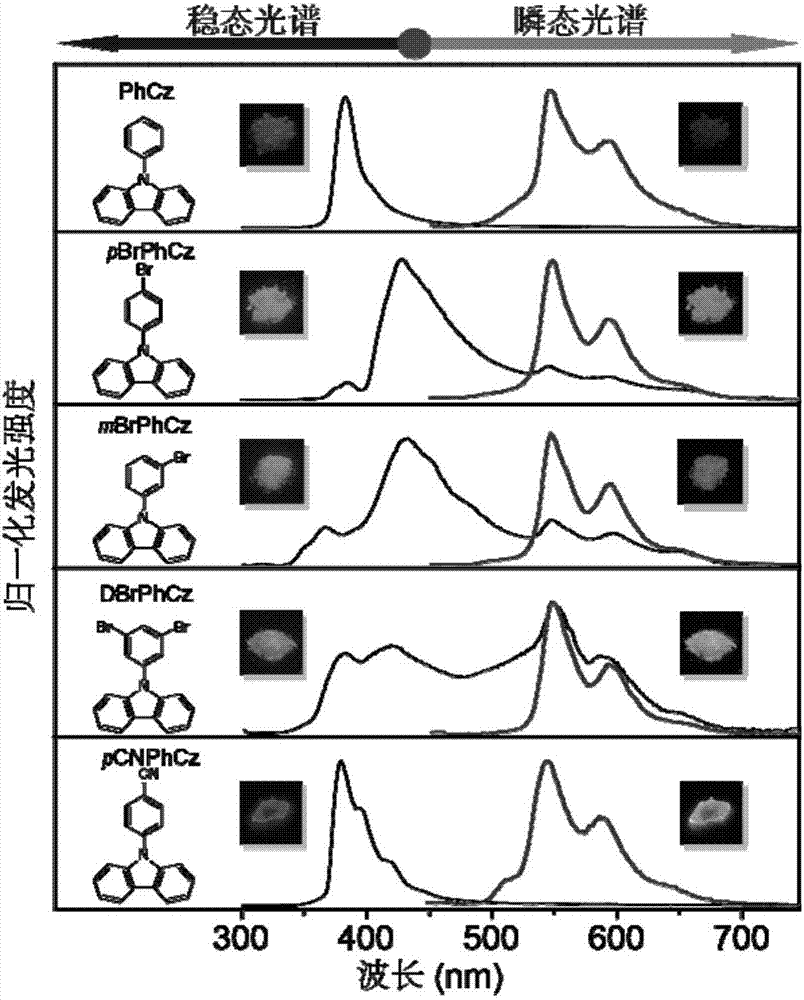
[0028] figure 1 It is the steady-state and transient spectral spectra of the obtained compound, the excitation wavelength is 295nm, and the transient spectral delay time is 10ms. The insets are the physical picture (left picture) and the a...
Embodiment 2
[0031] Synthesis of pBrPhCz: First, 1 times the equivalent of carbazole, 1 to 1.5 times the equivalent of p-dihalobenzene, such as p-dibromobenzene, 0.44 times the equivalent of Cu (electrolytically refined copper), 4.04 times the equivalent of K 2 CO 3 Put it into a reaction vessel, use nitrobenzene as a reaction solvent, and react under nitrogen protection at 120-160°C for 12-48 hours. The reaction liquid is distilled under reduced pressure to obtain a crude product, and a pure product is obtained by column chromatography with a yield of 75-85%.
[0032]
[0033] Its hydrogen spectrum 1 H NMR (400MHz, CDCl 3 ), δ (TMS, ppm): 8.16 (d, 2H), 7.75 (d, 2H), 7.47 (m, 6H), 7.34 (t, 2H).
[0034] figure 1 It is the steady-state and transient spectral spectra of the obtained compound, the excitation wavelength is 295nm, and the transient spectral delay time is 10ms. The insets are the physical picture (left picture) and the afterglow picture (right picture) of the correspondin...
Embodiment 3
[0037] Synthesis of mBrPhCz: First, 1 times the equivalent of carbazole, 1 to 1.5 times the equivalent of m-dihalobenzene, such as m-dibromobenzene, etc., 0.44 times the equivalent of Cu (electrolytic refined copper), 4.04 times the equivalent of K 2 CO 3 Put it into a reaction vessel, use nitrobenzene as a reaction solvent, and react under nitrogen protection at 120-160°C for 12-48 hours. The reaction liquid is distilled under reduced pressure to obtain a crude product, and a pure product is obtained by column chromatography with a yield of 75-85%.
[0038]
[0039] Its hydrogen spectrum 1 H NMR (400MHz, CDCl 3 ), δ (TMS, ppm): 8.30 (d, 2H), 7.90 (s, 1H), 7.71 (d, 1H), 7.62 (m, 8H).
[0040] figure 1It is the steady-state and transient spectral spectra of the obtained compound, the excitation wavelength is 295nm, and the transient spectral delay time is 10ms. The insets are the physical picture (left picture) and the afterglow picture (right picture) of the correspondi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com