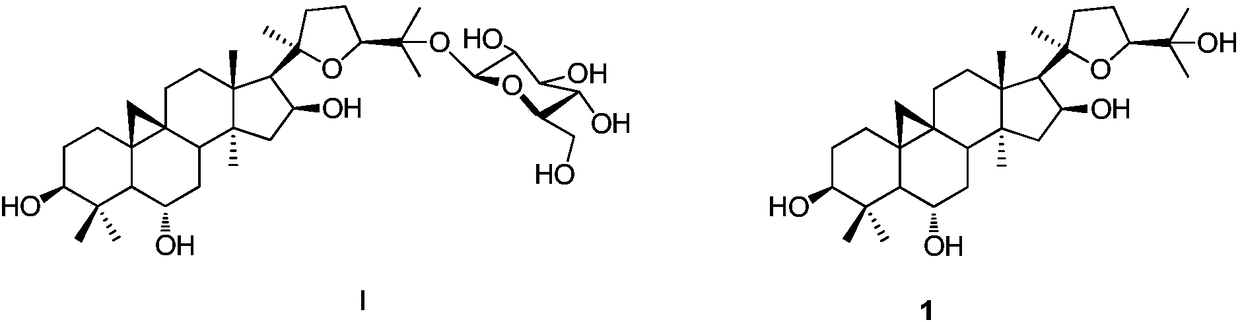
A kind of synthetic method of 25-o-glucosyl cycloastragenol
A cyclo-astragalus and glucose-based technology is applied in the field of preparation of 25-O-glucosyl cyclo-astragalus, which can solve the problems of unsuccessful synthesis of astragaloside, no reports of synthesis of astragalus, etc., and achieves the advancement of active mechanism research and improvement. The effect of its drug development process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
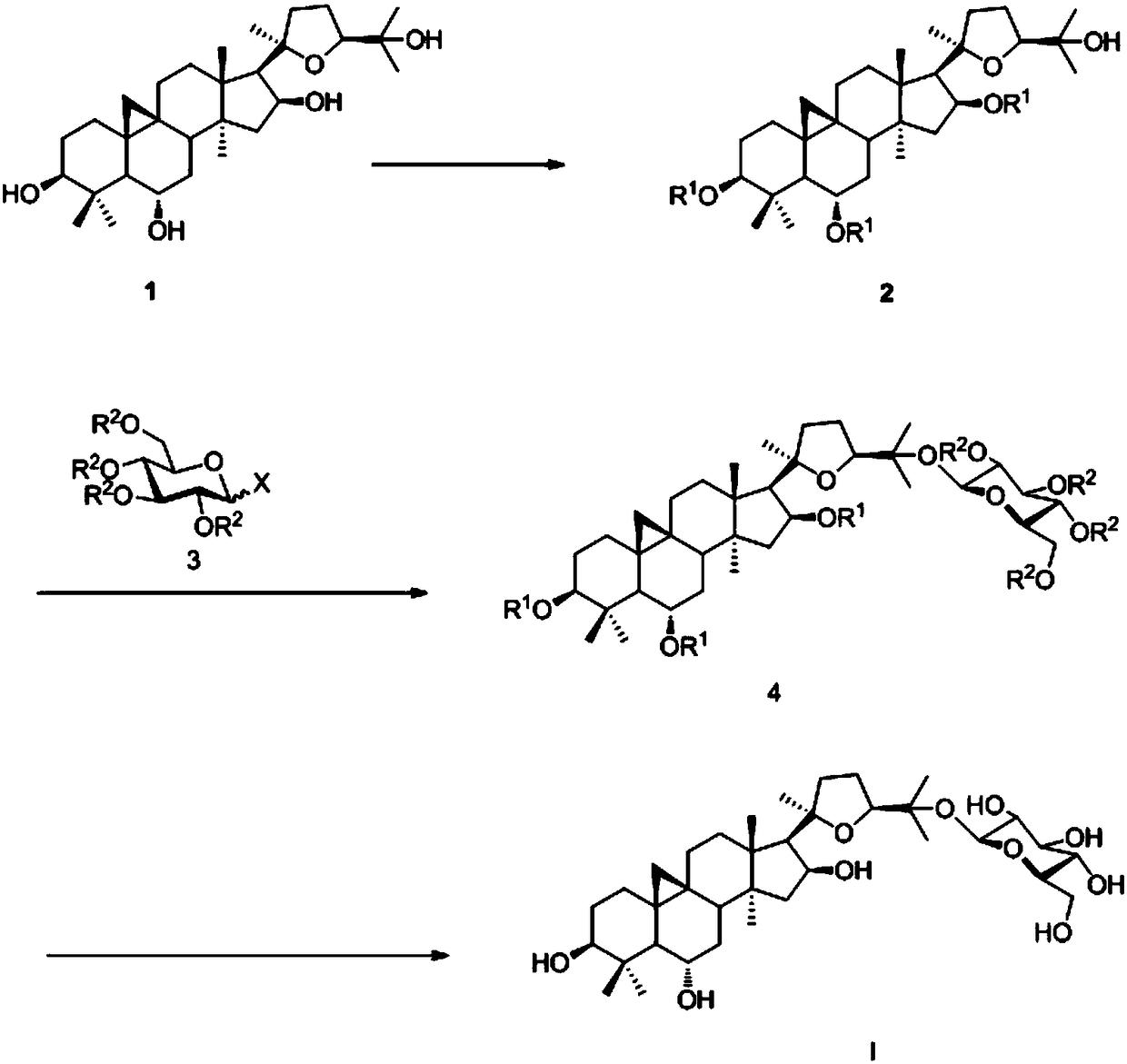
Embodiment 1
[0045] Example 1: Selective hydroxyl protection of cycloastragenol: Compound 2 was obtained;
[0046]
[0047] Under nitrogen protection, at 0°C, cycloastragenol (180mg, 0.37mmol), PPy (108mg, 0.74mmol) was dissolved in dry toluene (10mL), and acetic anhydride (348μL, 3.67mmol) was added to the system, DIPEA (912 μL, 5.55 mmol), the system was slowly raised to room temperature and then heated to 105 ° C, stirred until TLC tracking showed that the reaction of the raw materials was complete, the reaction system was extracted with ethyl acetate, and successively with 1mol / l HCl, saturated sodium bicarbonate , washed with saturated NaCl, dried over anhydrous sodium sulfate, filtered with suction, concentrated the crude product under reduced pressure, and then obtained white solid compound 2 (184mg, 81.3%) by column chromatography: [α] D 25 =73.5 (c=1, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ5.44-5.39(m,1H),4.75(td,J=4.4,9.2Hz,1H),4.60(dd,J=4.4,11.2Hz,1H),3.72(dd,J=6.8,8.4Hz ,1...
Embodiment 2
[0048] Example 2: Glycosidation reaction to obtain compound 4;
[0049]
[0050] Under nitrogen protection, compound 2 (12 mg, 0.02 mmol) and glucosynyl ester donor 3 (31 mg, 0.05 mmol) were dissolved in dry dichloromethane (2 mL), and 4A molecular sieves (2 mg) were added, at room temperature Stir for half an hour before adding the catalyst Ph 3 PAuNTf2 (0.002mmol), continued to stir at room temperature until TLC tracking showed that the reaction of raw materials was complete, the crude product was concentrated under reduced pressure, and then column chromatography gave white solid compound 4 (22mg, 94.42%): [α] D 25 =72.6 (c=1, CHCl 3 ); 1 HNMR (400MHz, CDCl 3 )δ8.00-7.95(m,4H),7.91(dd,J=1.2,8.4Hz,2H),7.85(dd,J=1.2,8.0Hz,2H),7.56-7.48(m,3H),7.44 -7.33(m,7H),7.30-7.27(m,2H),5.92(t,J=9.6Hz,1H),5.58(t,J=10.0Hz,1H),5.50(dd,J=8.0,10.0 Hz, 1H), 5.29-5.26(m, 1H), 5.20(d, J=8.0Hz, 1H), 4.74(td, J=4.0, 9.2Hz, 1H), 4.60-4.56(m, 2H), 4.47 (dd, J=6.8, 12.0Hz, 1H), 4.16-4.11(m,...
Embodiment 3
[0051] Example 3: Final deprotection reaction: 25-O-glucosyl cycloastragenol I was obtained;
[0052]
[0053] Under the protection of nitrogen, compound 4 (20mg, 0.017mmol) was dissolved in dry methanol (2mL), then sodium methoxide (0.17mmol) was added, and stirred at room temperature until TLC tracking showed that the reaction of the raw material was complete, and then neutralized with an acidic resin PH to neutral and slightly acidic, suction filtered, concentrated the crude product under reduced pressure, and then column chromatography to obtain the white solid compound 25-O-glucosyl cycloastragenol I (9.2mg, 84%): [α] D 25 =21.4 (c=1, CHCl 3 ); 1 H NMR (400MHz, C 5 D. 5 N)δ5.10(d,J=8.0Hz,1H),4.95-4.88(m,2H),4.47(dd,J=2.8,11.6Hz,1H),4.34(dd,J=4.8,11.6Hz, 1H), 4.25-4.16(m, 2H), 4.09(dd, J=9.2, 16.0Hz, 1H), 3.93-3.89(m, 2H), 3.83-3.77(td, J=4.4, 10.0Hz, 1H) ,3.70(dd,J=4.4,11.2Hz,1H),2.87-2.80(m,1H),2.47(d,J=7.6Hz,1H),1.92(s,3H),1.68(s,3H), 1.43(s,3H),1.38(s,6H),1.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com