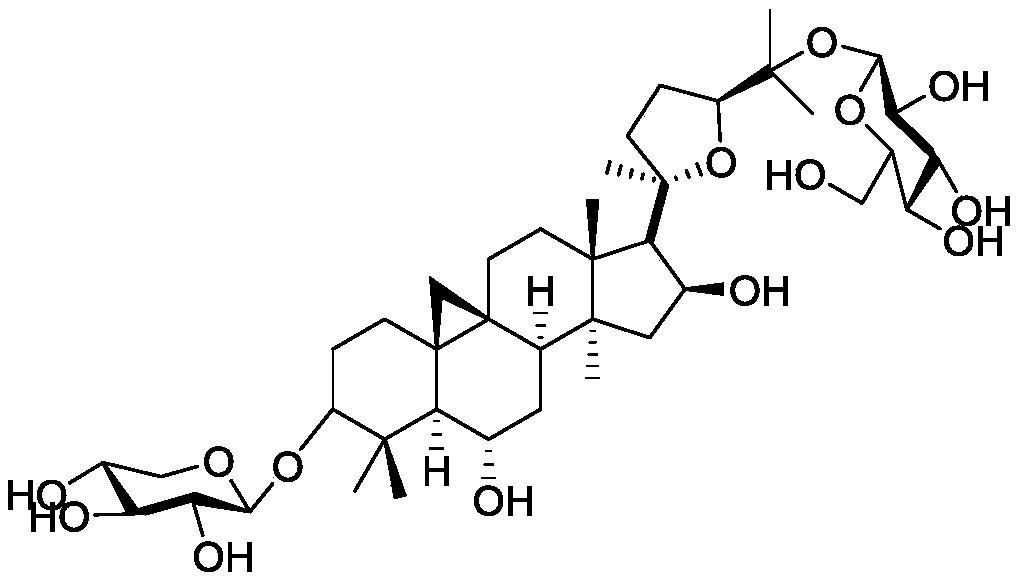
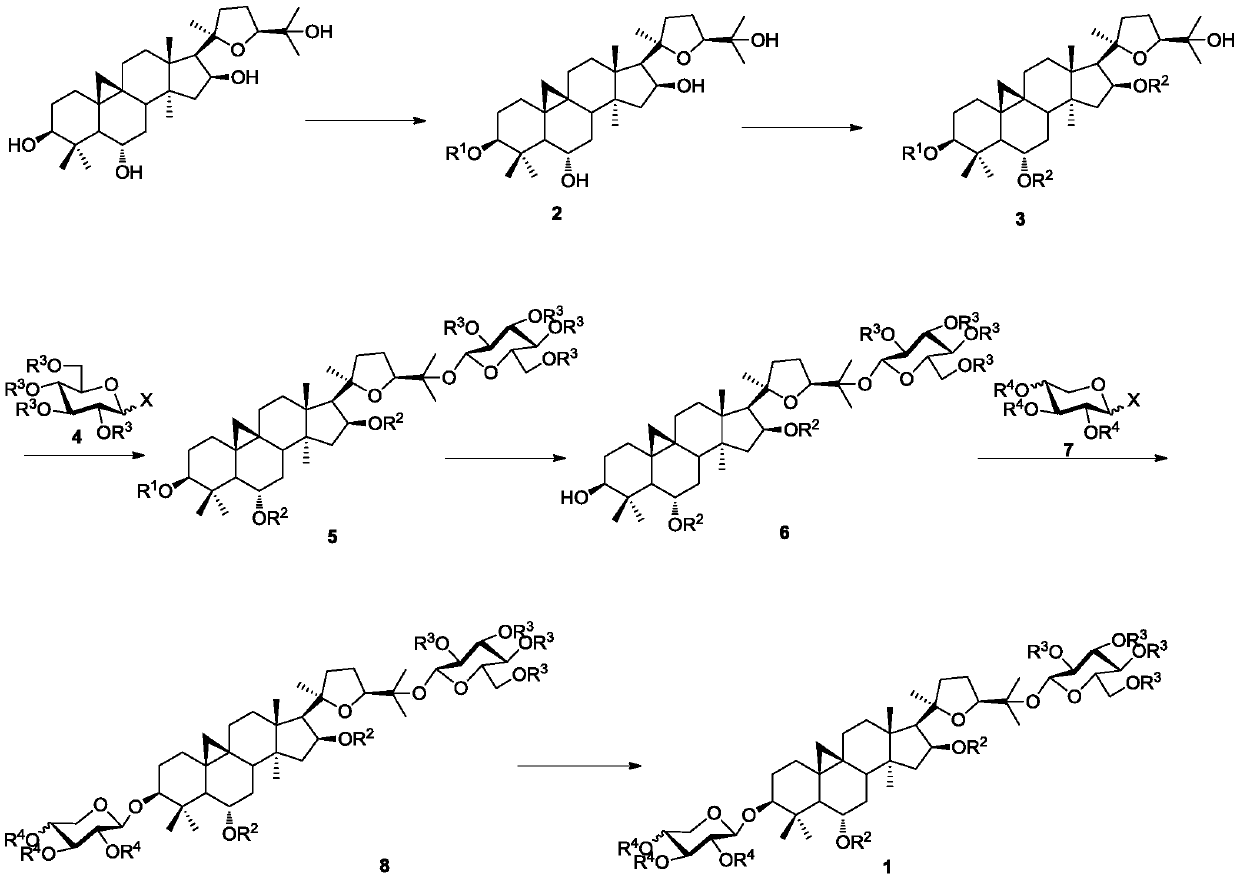
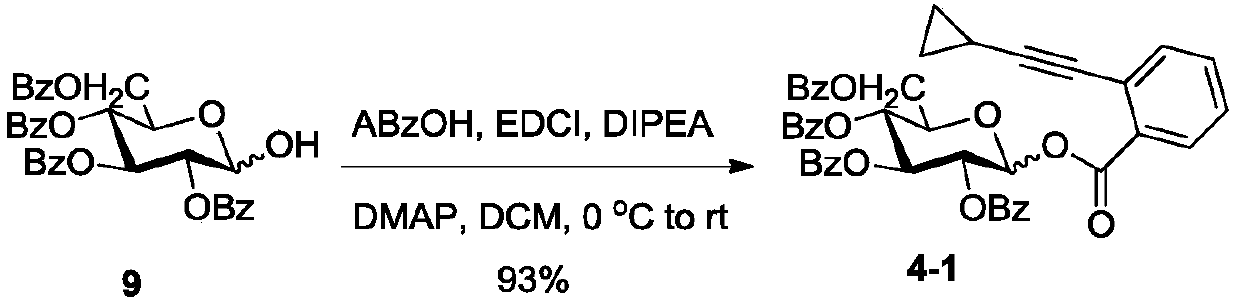
A kind of synthetic method of isoastragaloside IV
A technique for the synthesis of astragaloside IV, which is applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve problems such as failure to find success and difficulty in synthesizing isoastragloside IV, and promote the research on the mechanism of activity and its medicine Progress of development, effect of high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of isoastragaloside IV,
[0040]
[0041] (1) Synthesis of cycloastragenol derivative 2 protected by the 3-hydroxyl group,
[0042]
[0043] Under nitrogen protection, cycloastragenol (400mg, 0.82mmol) was dissolved in dry DMF, and TBSCl (489mg, 3.3mmol) and imidazole (333mg, 4.9mmol) were added to the system, and stirred at room temperature until TLC tracking showed that the reaction of the raw materials was complete , the reaction system was extracted with ethyl acetate, washed successively with 1mol / l HCl, saturated sodium bicarbonate, and saturated NaCl, dried over anhydrous sodium sulfate, filtered with suction, concentrated the crude product under reduced pressure, and then obtained a white solid compound by column chromatography 2 (76.8%): [α] D 25 =30.5 (c=1, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ4.67(dd, J=7.6,14.4Hz,1H),3.72(dd,J=6.0,8.0Hz,1H),3.51(td,J=3.6,9.6Hz,1H),3.25(dd,J =4.8,10.0Hz,1H),2.59(dd,J=10.4,21.6Hz,1H),2.31(d,J=8.0Hz,1H),2.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com