Supermolecular bactericide as well as preparation method and sterilization method thereof
A supramolecular and fungicide technology, applied in the field of supramolecular chemistry, can solve the problems affecting the activity and effect of fungicides, and achieve the effect of delaying drug-resistant pathogens and avoiding long-term coexistence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4 and comparative example 1-4
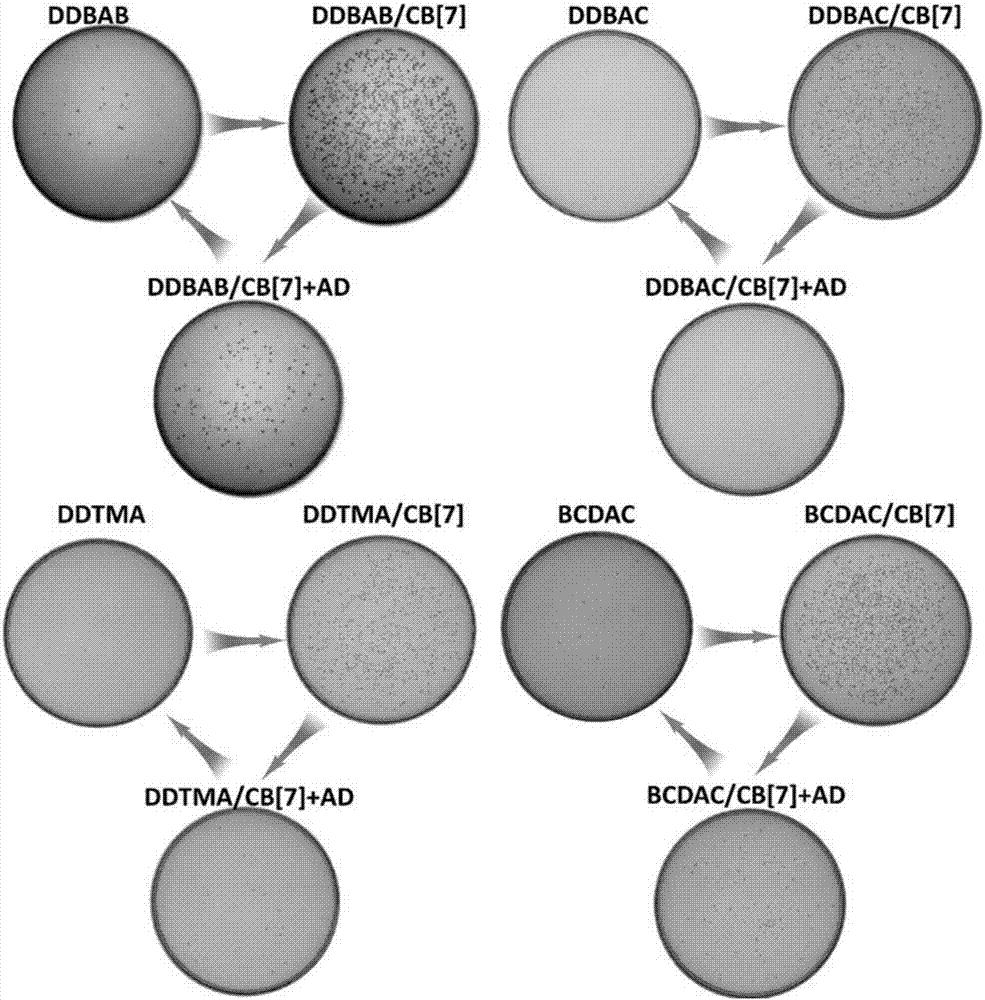
[0057] (1) Preparation of fungicides
[0058] Weigh the cationic surfactant represented by the formula (I) and the cucurbit [n] urea represented by the formula (II), and completely dissolve them in 5 mL of ultrapure water at 40° C. to prepare a fungicide with a concentration of 2 mM.
[0059] Table 1 shows the material types, concentrations, material combination ratios, combination constants and obtained bactericide structures used in the fungicide systems of Preparation Examples 1-4 and Comparative Examples 1-4. Wherein, the bactericide that embodiment 1-4 obtains is supramolecular bactericide provided by the present invention, does not add cucurbit [n] urea in comparative example 1-4, and the bactericide that obtains is the cationic surface shown in formula (I) active agent.
[0060] Table 1
[0061]
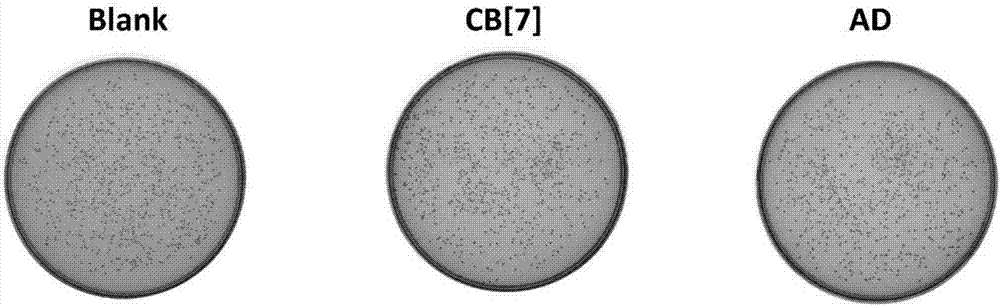
[0062] (2) The inhibitory effect of fungicides on pathogenic bacteria
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com