Vincristine sulfate liposome and its preparation method and application
A technology of vincristine sulfate and liposomes, which is applied in the directions of liposome delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of low stability of vincristine sulfate liposomes, etc. Anti-tumor effect, improving drug enrichment, protecting and stabilizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
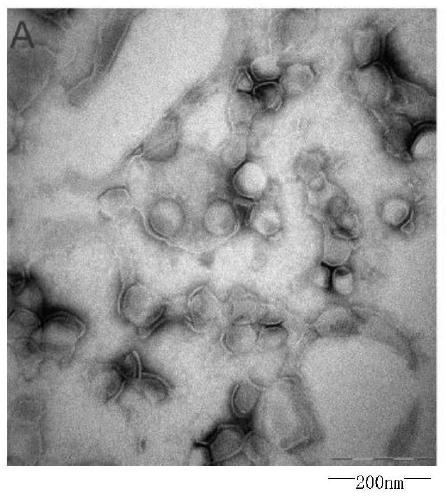
Image
Examples
preparation example Construction
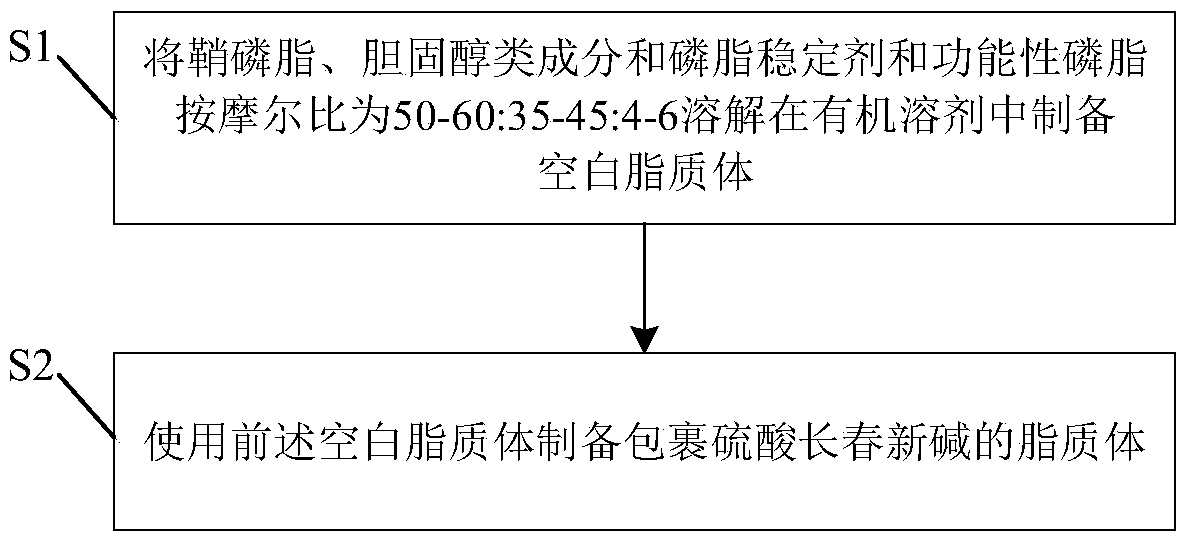
[0033] The present invention also provides the preparation steps and parameters of suitable vincristine sulfate liposomes especially for sphingomyelin, this is because the chemical structure and hydrophilicity of different phospholipids are different, and the performance of the drug wrapped is different, resulting in the present The steps and parameters for preparing and encapsulating antitumor drugs with other phospholipids in the prior art are not fully applicable to the present invention. see figure 1 , for the preparation method of the vincristine sulfate liposome according to the preferred embodiment of the present invention, the vincristine sulfate liposome as described above can be prepared. As shown in the figure, the preparation method includes:
[0034]First, in step S1, the sphingomyelin component, the cholesterol component and the phospholipid stabilizer are dissolved in an organic solvent in a molar ratio of 50-60:35-45:4-6 to prepare a blank liposome. Preferabl...
Embodiment 1
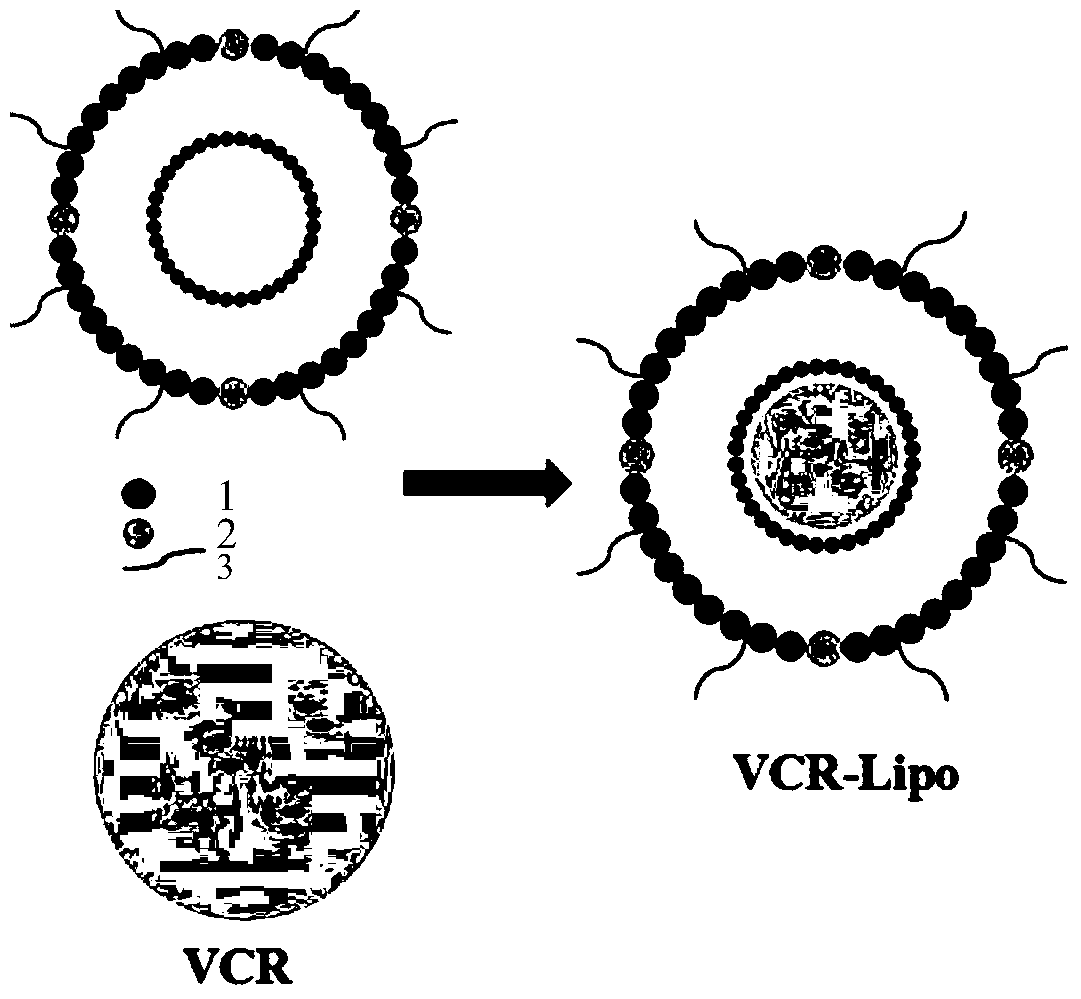
[0049] This embodiment 1 prepares vincristine sulfate liposome VCR-Lipo.
[0050] Preparation of S1, PEG long-circulating liposome (Lipo):
[0051] Egg sphingomyelin (SM), cholesterol (Cholesterol), and DSPE-PEG2000 dissolved in chloroform were added into a round-bottomed flask at a molar ratio of 55:40:5, and a rotary evaporator was used at 60°C at a speed of Rotate at 200rpm / min and turn on the vacuum pump to vacuum for 10min to form a dry film, and dry overnight in a vacuum dryer to remove residual organic solvents.
[0052] Among them, SM is the product of Avanti polar lipids company, the product number is 860061P; cholesterol is the product of Avantipolar lipids company, the product number is 700100P; DSPE-PEG2000 is the product of Avanti polar lipids company, the product number is 880128P.
[0053] The lipid film at the bottom of the bottle was hydrated with 1 ml of 300 mM citrate buffer (pH=4.0), and after ultrasonication in a 50°C water bath for 20 min, it was repeate...
Embodiment 2
[0057] This embodiment 2 prepares vincristine sulfate liposome VCR-Lipo.
[0058] Preparation of S1, PEG long-circulating liposome (Lipo):
[0059]Egg sphingomyelin (SM), cholesterol (Cholesterol), and DSPE-PEG2000 dissolved in chloroform were added into a round-bottomed flask in a molar ratio of 50:45:4. Rotate at 100rpm / min and turn on the vacuum pump to vacuum for 20min to form a dry film, and dry overnight in a vacuum dryer to remove residual organic solvents.
[0060] Wherein raw material SM, cholesterol and the product that DSPE-PEG2000 selects for use are identical with embodiment 1.
[0061] The lipid film at the bottom of the bottle was hydrated with 1 ml of 300 mM citrate buffer (pH=4.0), and after ultrasonication in a 60°C water bath for 30 min, it was repeatedly frozen and thawed 6 times in liquid nitrogen and a 50°C water bath. The product is packed into a liposome pusher, passed through the polycarbonate membrane of 200nm and 100nm successively for 25 times, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com