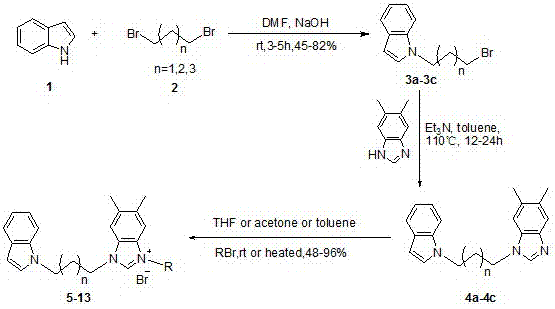
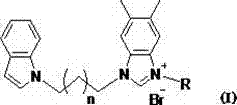
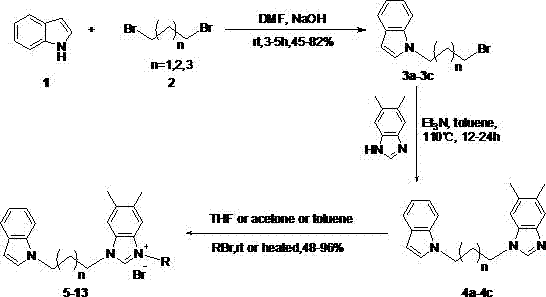
N-alkyl substituted indole-imidazolium salt compounds, and preparation method thereof
A salt compound and compound technology, applied in organic chemistry, drug combination, pharmaceutical formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method specifically includes:
[0022] A, the preparation of compound N-alkyl substituted indole:
[0023] Using indole as raw material, synthesize N-alkyl substituted indole with dibromoalkane in anhydrous DMF solvent: dissolve indole in anhydrous DMF, add sodium hydroxide solid at 0 ℃, the amount is molar ratio For indole: sodium hydroxide = 1: 2, the dosage of anhydrous DMF is 20 ml: 1g indole, stir for 5 minutes, then add dibromoalkane (1,3-dibromopropane, 1,4- Dibromobutane or 1,5-dibromopentane) in anhydrous DMF, the molar ratio is dibromoalkane:indole=3:1, the amount of anhydrous DMF is 5 ml : 1 ml dibromoalkane , after stirring and reacting at room temperature for 5 hours, dilute with ethyl acetate (50 ml: 1g substrate), wash with water (50 ml) and saturated brine (50 ml) respectively, and wash the organic phase with anhydrous Na 2 SO 4 After drying, filtering, and concentrating the solvent under reduced pressure, silica gel column chromatogra...
Embodiment 1
[0029] Preparation of Compound 5: See Preparation Methods A, B, and C above.
[0030]
[0031] Compound 5: Formula C 27 h 27 Br 2 N 3 , yield 69%. White solid, m.p. 108-109 o C. IR ν max (cm -1 ): 3446, 3129, 2361, 2052, 1611, 1562, 1445, 1384, 1312, 1268, 1222, 1129, 1069, 1024, 957, 860, 743, 616, 563, 428. 1 H NMR (400 MHz, DMSO): δ9.72 (1H, s), 7.75 (2H, s), 7.68 (1H, s), 7.55-7.53 (1H, d, J =7.37 Hz),7.49-7.47 (1H, d, J =7.69Hz),7.41-7.35(3H,m),7.29-7.27(1H,d, J =6.73Hz), 7.12-7.09 (1H, t, J = 14.11 Hz), 7.03-7.00 (1H, t, J = 13.47 Hz), 6.43 (1H, s), 5.71 (2H, s), 4.52 (2H, s), 4.36 (2H, s), 2.43 (2H, s), 2.35 (6H, s). 13 C NMR(100 MHz, DSMO): δ 141.82, 136.61, 136.54, 135.57, 133.26, 132.87, 130.92,130.37, 129.64, 129.56, 128.58, 128.47, 128.30, 128.16, 122.91, 121.14,120.55, 119.14, 113.34, 113.17, 109.87, 100.89, 50.20, 44.66, 42.80, 29.22, 20.08, 20.01.
Embodiment 2
[0033] Preparation of Compound 6: See Preparation Methods A, B, and C above.
[0034]
[0035] Compound 6: Molecular Formula C 27 h 28 BrN 3 , Yield 63%. White solid, m.p.164-165 o C.IRν max (cm -1 ):3448, 3128, 2320, 2051, 1644, 1561, 1456, 1354, 1262, 1128, 1069, 994, 955,861, 743, 614, 541. 1 HNMR(400MHz,DMSO):δ9.79(1H,s),9.48(1H,s),7.71 (1H,s),7.67 (1H,s), 7.55-7.53 (1H,d, J = 7.66 Hz), 7.48-7.46 (1H, d, J = 8.06 Hz),7.41-7.40 (1H, d, J = 3.07 Hz), 7.39 (1H, s), 7.37 (1H, s), 7.21 (1H, s),7.19 (1H, s), 7.13-7.09 (1H, m), 7.04-7.00 (1H, m) , 6.43-6.42 (1H, d, J =3.04 Hz), 5.59 (2H, s), 4.48-4.44 (2H, t, J= 14.76 Hz), 4.38-4.34 (2H, t, J = 13.75 Hz), 2.47-2.43 (2H, t, J = 14.29 Hz), 2.35 (3H, s), 2.34 (3H, s),2.27 (3H, s). 13 C NMR (100 MHz, DSMO): δ 141.11, 138.04, 136.28, 135.53,131.07, 129.63, 129.44, 129.29, 128.45, 128.19, 128.13, 121.07, 120.49,119.07, 113.20, 113.08, 109.75, 100.87, 49.45, 44.51, 42.75, 29.01, 20.69, 19.99, 19.92.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com