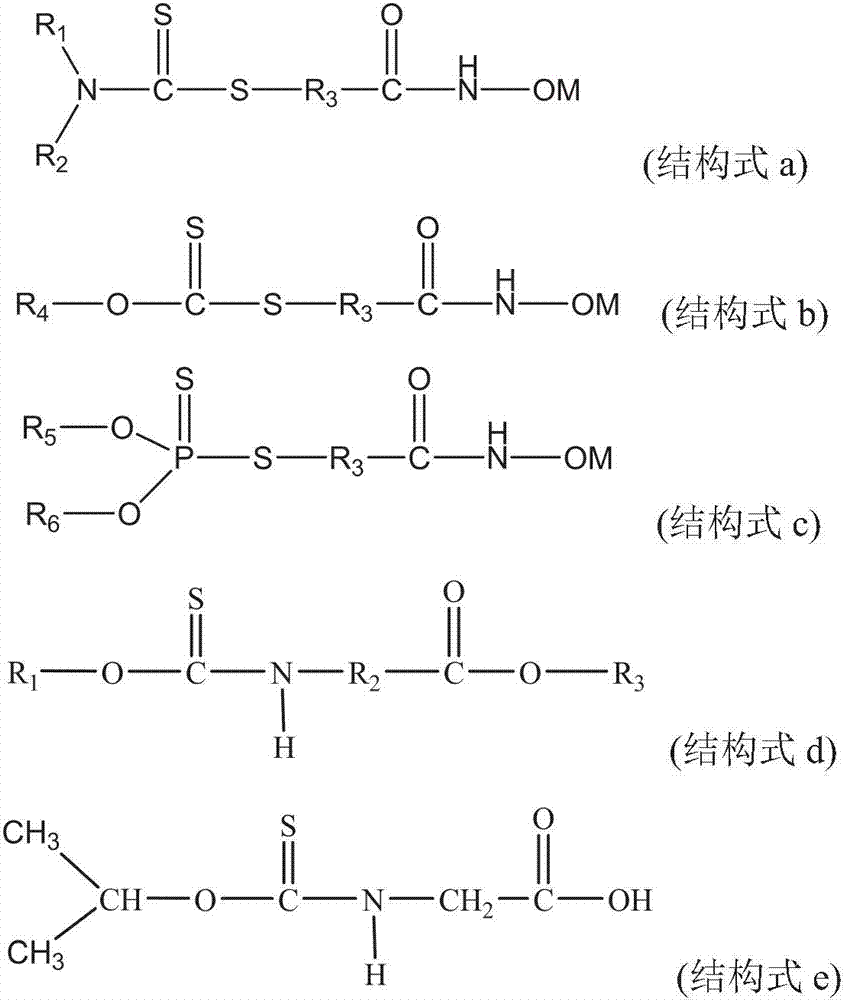
N-alkylhydroxyoxime acid-O-alkyl thiamine collecting agent, preparation and application of N-alkylhydroxyoxime acid-O-alkyl thiamine collecting agent
A technology of hydrocarbyl thiourethane and alkyl hydroxamic acid, which is applied in the direction of solid separation and flotation, can solve the problem of no N-alkyl, etc., achieve good overall performance, improve flotation recovery rate, and strengthen hydrophobicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of N-(4-butylhydroxamic acid)-O-dodecylthiocarbamate
[0058] Dissolve 11.65 parts of sodium chloroacetate in 60 parts of water, add 29.82 parts of sodium n-dodecyl xanthate under stirring, then add a small amount of sodium hydroxide to adjust the pH of the solution to about 7.5, and react in a water bath at 80°C After 2 hours, cool down to room temperature, add 16.22 parts of 4-aminobutyroxamic acid under stirring, and then react at 70°C for 1 hour, stop stirring, cool down to room temperature, there is a white solid, filter, and the obtained white solid is the desired Product, the yield based on sodium chloroacetate is 93.6%.
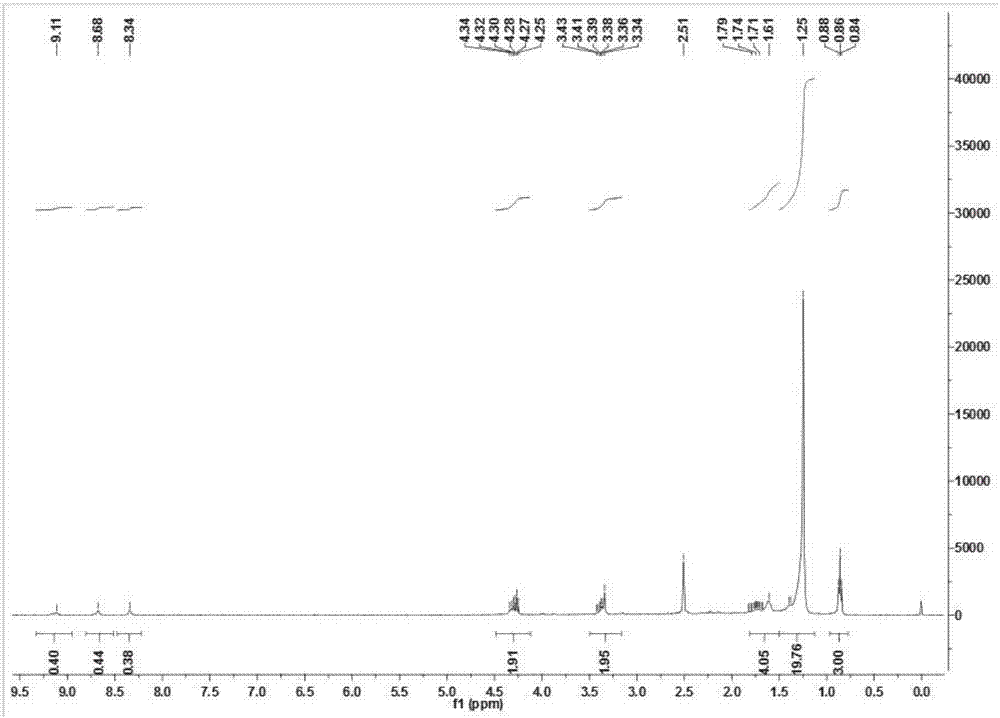
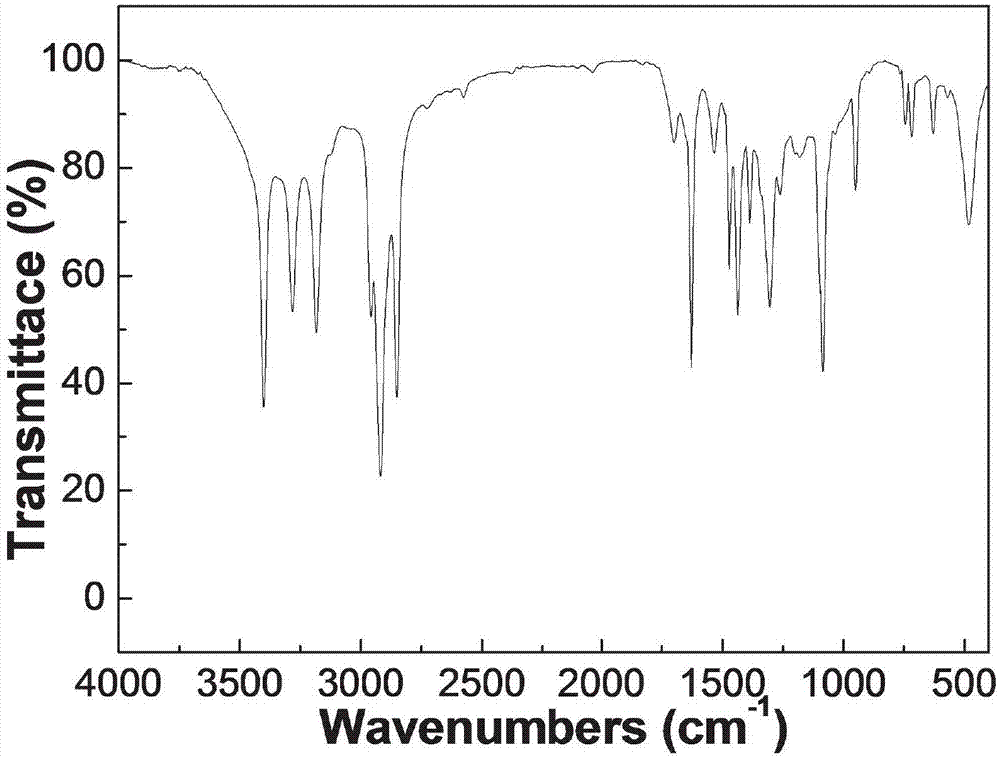
[0059] The hydrogen spectrum data of the N-(4-butylhydroxamic acid)-O-dodecylthiocarbamate obtained in this embodiment is shown in Table 1: the infrared spectrum information is shown in Table 2:
[0060] Table 1
[0061]
[0062] Table 2
[0063]
Embodiment 2
[0065] Preparation of N-(4-butylhydroxamic acid)-O-octylthiocarbamate
[0066] Dissolve 11.65 parts of sodium chloroacetate in 60 parts of water, add 23.94 parts of n-octyl xanthate sodium under stirring, then add a small amount of sodium hydroxide to adjust the pH of the solution to about 7.5, and react in a water bath at 80°C for 2 hours , cool down to normal temperature, add 15.82 parts of 4-aminobutyroxamic acid under stirring, and then react at 70°C for 1 hour, stop stirring, let stand to separate layers, the upper layer of oily liquid is the desired product, based on sodium chloroacetate The yield was 92.4%.
Embodiment 3
[0068] Preparation of N-(6-hexylhydroxamic acid)-O-octylthiocarbamate
[0069] Dissolve 11.65 parts of sodium chloroacetate in 60 parts of water, add 23.94 parts of sodium n-octyl xanthate under stirring, then add a small amount of sodium hydroxide to adjust the pH of the solution to about 7.5, and react in a water bath at 80°C for 2.5 hours , cool down to normal temperature, add 19.16 parts of 6-aminocaproxamic acid under stirring, and then react at 70°C for 1.5 hours, stop stirring, stand and separate layers, the upper oily liquid is the desired product, based on sodium chloroacetate The yield was 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com