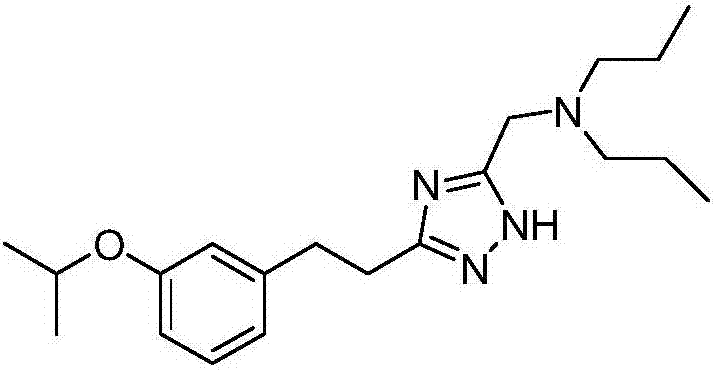
Preparation method of azole compound
A technology of azole compounds and compounds is applied in the field of novel preparation of pharmaceutical intermediates, and can solve problems such as difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
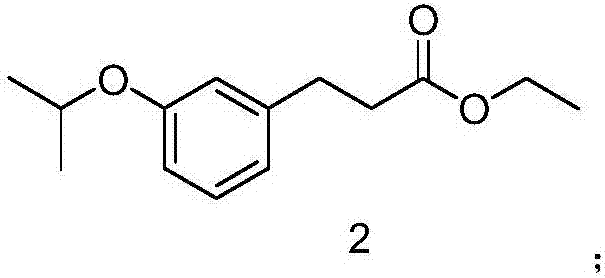
[0026] (1) Synthesis of 3-(3-isopropoxyphenyl) ethyl propionate
[0027] Add 25g of 3-(3-isopropoxyphenyl)ethyl acrylate to 260ml of methanol, add 19g of sodium borohydride, stir at room temperature for 3 hours, cool, concentrate, add water and ethyl acetate, extract and separate, and collect The organic phase was dried and concentrated to yield 19 g of ethyl 3-(3-isopropoxyphenyl)propionate.
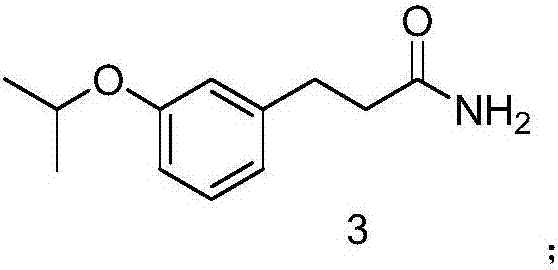
[0028] (2) Synthesis of 3-(3-isopropoxyphenyl) propanamide
[0029] Add 19g of ethyl 3-(3-isopropoxyphenyl) propionate to 600ml of ammonia water, then add 200ml of water, heat to 80°C, stir for 10 hours, add ethyl acetate to extract and separate the liquid, collect the organic phase, Drying and concentration gave 16 g of 3-(3-isopropoxyphenyl)propanamide.
[0030] (3) Synthesis of 3-(3-isopropoxyphenyl) propionimidate
[0031] Add 15g of 3-(3-isopropoxyphenyl)propionamide to 180ml of tetrahydrofuran, slowly add 11g of triethyloxonium tetrafluoroboric acid, heat and reflux and stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com