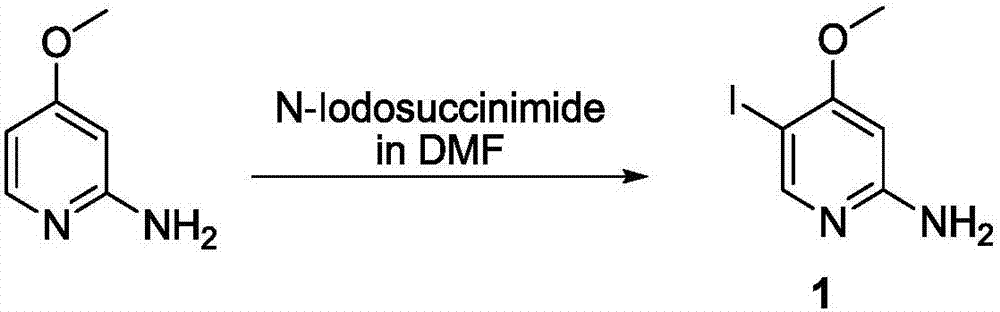
Method for synthesizing 5-iodo-4-methoxy-2-aminopyridine
A technology of methoxypyridine and aminopyridine is applied in the synthesis field of 5-iodo-4-methoxy-2-aminopyridine, and can solve the problem that there is no public report on the synthesis method of N-iodosuccinimide, Expensive reagents, high reaction temperature, etc., to achieve the effects of low cost, short reaction time and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 2-amino-4-methoxypyridine (7.8g, 62.9mmol) and N,N-dimethylformamide (150mL) into a three-necked flask; add N-iodosuccinimide ( 14.1 g, 62.7 mmol). The reaction solution was stirred at 25°C for 60 minutes. The reaction solution was diluted with water (400mL), filtered to obtain a solid crude product, and separated by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain a brown solid, the target product 5-iodo-4-methoxy-2- Aminopyridine (7.24 g, 28.9 mmol, 46%). The NMR results are as follows: 1 H NMR (400MHz, CDCl 3 ):8.16(s,1H),5.98(s,1H),4.44(br,2H),3.86(s,3H)ppm. Liquid chromatography-mass spectrometry detection results are as follows: LC-MS(ESI):m / z 250.90[M+H] + .
Embodiment 2
[0019] Step 1: The reaction time of 2-amino-4-methoxypyridine and N-iodosuccinimide in N,N-dimethylformamide is 30 minutes, the reaction temperature is 50°C, and the reaction solution is water ( 400mL), filtered to obtain the solid crude product, separated by silica gel column chromatography (dichloromethane:methanol=20:1), to obtain a brown solid, the target product 5-iodo-4-methoxy-2-aminopyridine (yield rate 45.2%). The NMR results are as follows: 1 H NMR (400MHz, CDCl 3 ):8.16(s,1H),5.98(s,1H),4.44(br,2H),3.86(s,3H)ppm. Liquid chromatography-mass spectrometry detection results are as follows: LC-MS(ESI):m / z250.90[M+H] + .
Embodiment 3
[0021] Step 1: The reaction time of 2-amino-4-methoxypyridine and N-iodosuccinimide in N,N-dimethylformamide is 90 minutes, the reaction temperature is 0°C, and the reaction solution is water ( 400mL), filtered to obtain the solid crude product, separated by silica gel column chromatography (dichloromethane:methanol=20:1), to obtain a brown solid, the target product 5-iodo-4-methoxy-2-aminopyridine (yield rate 45.5%). The NMR results are as follows: 1 H NMR (400MHz, CDCl 3 ):8.16(s,1H),5.98(s,1H),4.44(br,2H),3.86(s,3H)ppm. Liquid chromatography-mass spectrometry detection results are as follows: LC-MS(ESI):m / z250.90[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com