Patents
Literature
35 results about "N-iodosuccinimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
N-Iodosuccinimide 95% CAS Number 516-12-1. Empirical Formula (Hill Notation) C 4 H 4 INO 2. Molecular Weight 224.98 . Beilstein Registry Number 113917 . EC Number 208-221-6. MDL number MFCD00005512. PubChem Substance ID 24853162
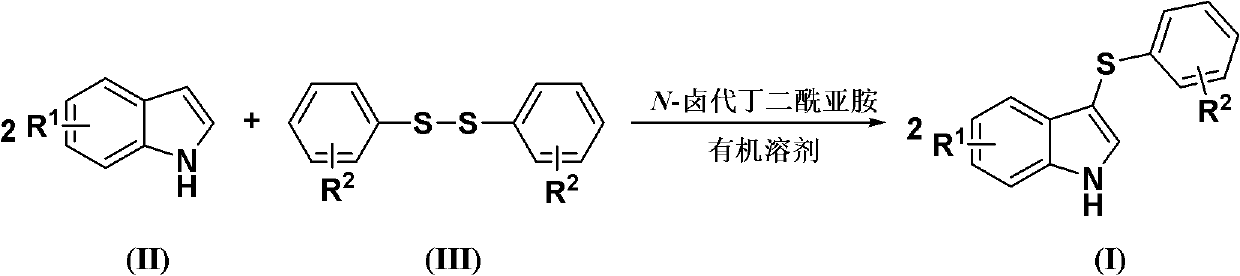
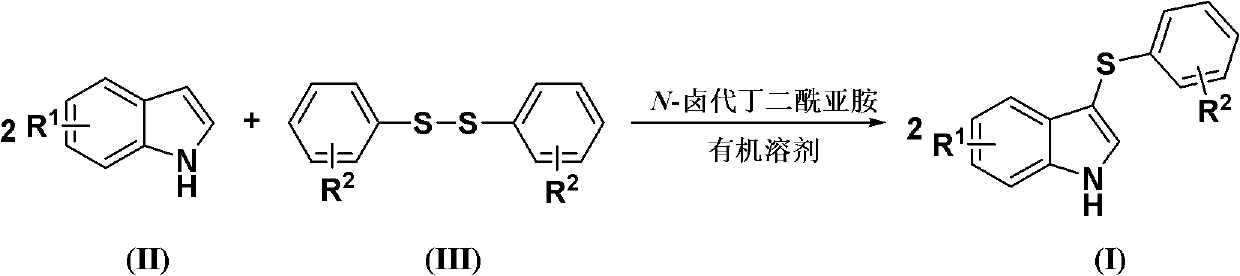
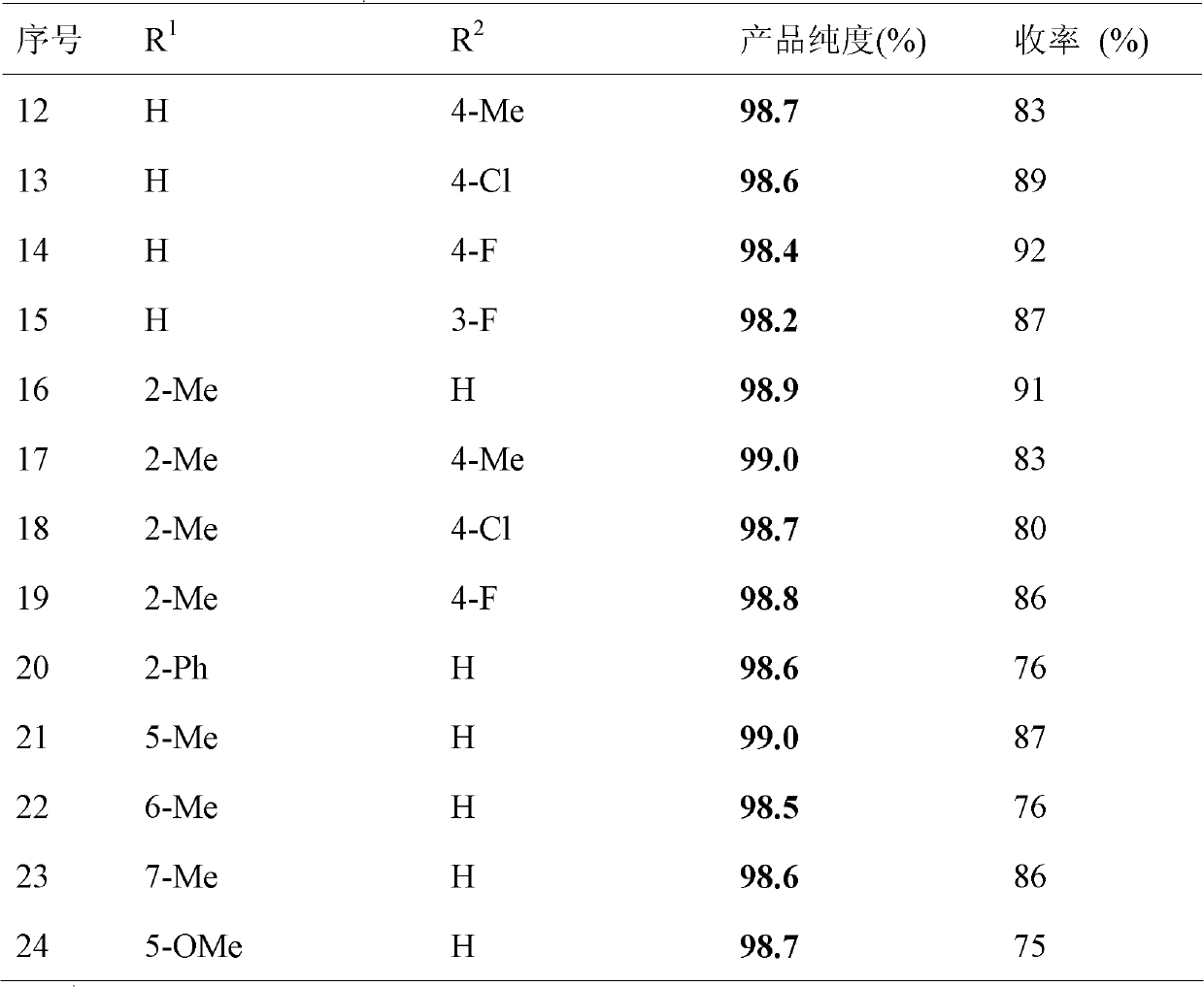
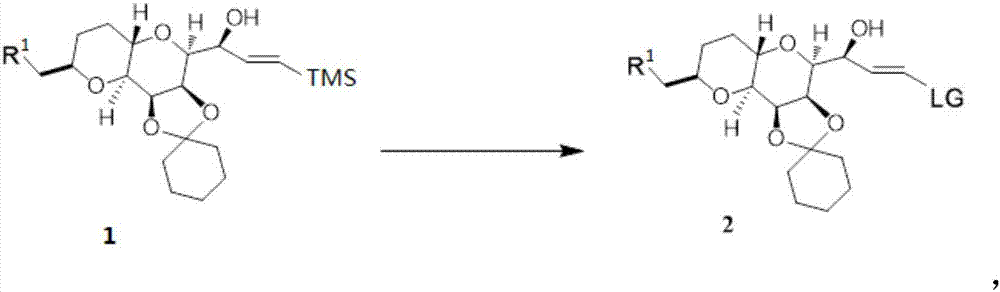
Method for synthesizing 3-aryl sulfydryl indole compound
The invention discloses a method for synthesizing a 3-aryl sulfydryl indole compound which is shown as a formula (I). According to the method for synthesizing the 3-aryl sulfydryl indole compound, an indole compound which is shown as a formula (II) and diaryl disulfides which are shown as a formula (III) are taken as raw materials, and the 3-aryl sulfydryl indole compound is obtained by fully reacting the indole compound and the diaryl disulfides in an organic solvent at the temperature of between -30 and 40 DEG C in the presence of N-halogenate succinimide; and the N-halogenate succinimide is one of N-chlorobutanimide, N-bromosuccinimide, and N-Iodosuccinimide. According to the method, a nonmetal low-toxicity reaction system is used, and cost is low; reaction selectivity and yield are high; a process route is advanced and reasonable, and reaction conditions are mild; and the method has great implementation value and social and economic benefit.
Owner:WENZHOU UNIVERSITY
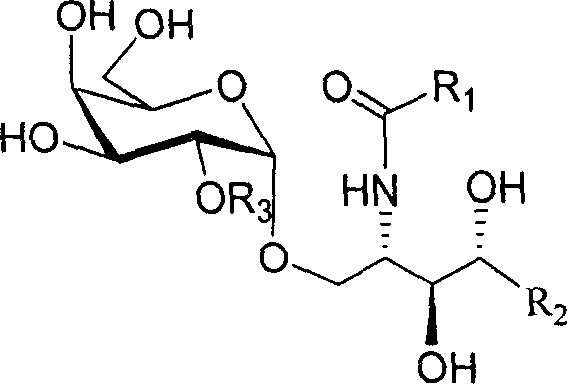
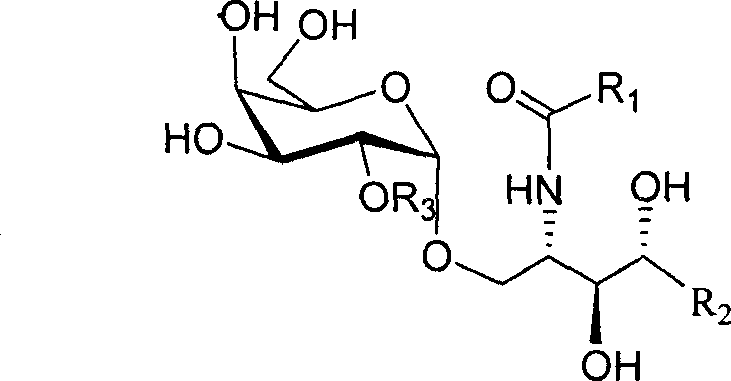
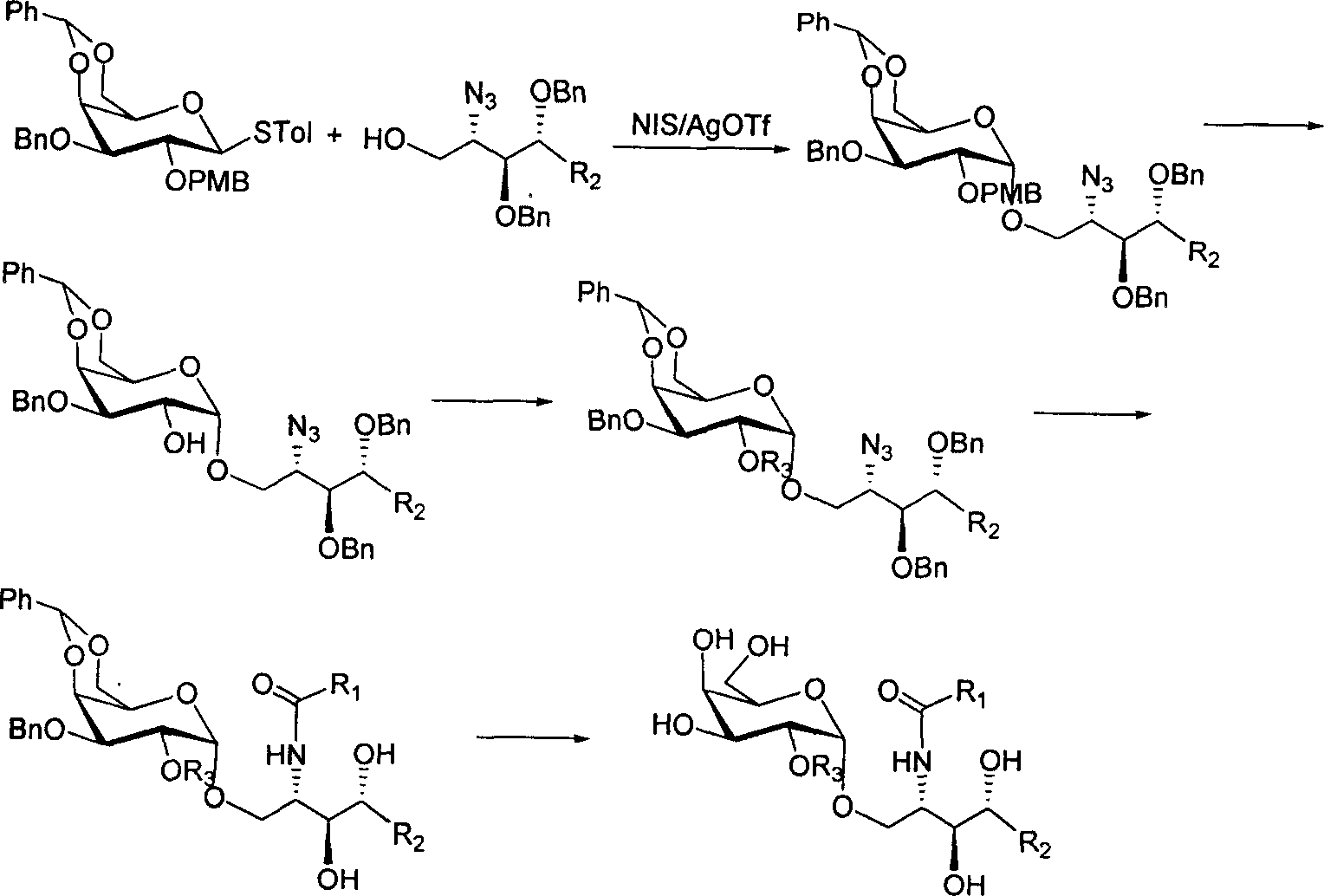
2'-OH derivative of alpha-galactose glycosphingolipids and preparing method thereof
The invention discloses a making method of 2'-OH derivant of alpha-galactose sphislycolipid, which comprises the following steps: synthesizing intermediate of alpha--galactose sphislycolipid through 3-O-benzyl-4, 6-O-benzal-2-O-p-methoxybenzyl p-methyl bentiamine and plant sphingol under N-iodosuccinimide and catalyst; introducing C18 alkyl, methyl, prenyl or alpha / beta connected galactose radical into naked -OH derivant on the intermediate without 2' p-methoxybenzyl; reducing azido group into amino group; acylating with aliphatic acid acted by accelerator; hydrogenating through palladium agent or liquid amino-metal sodium to reduce.
Owner:OCEAN UNIV OF CHINA
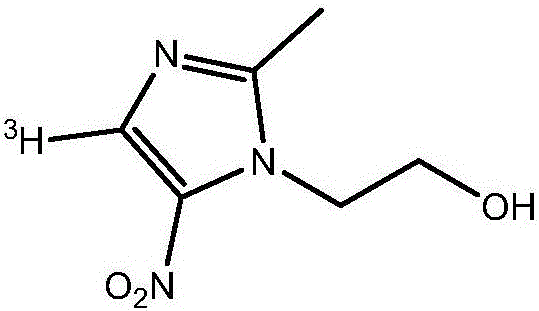
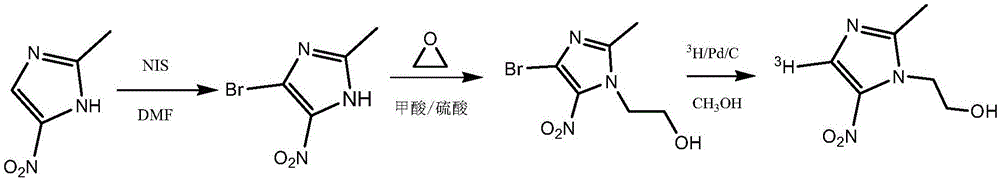
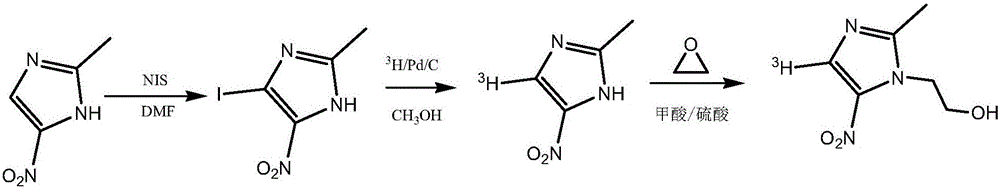
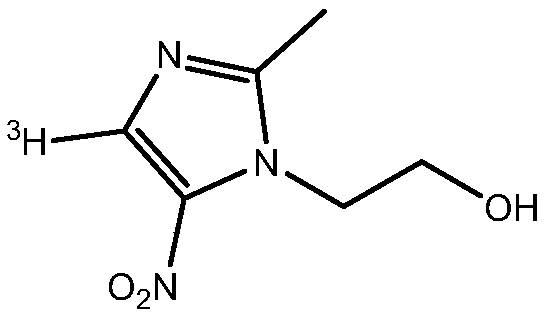
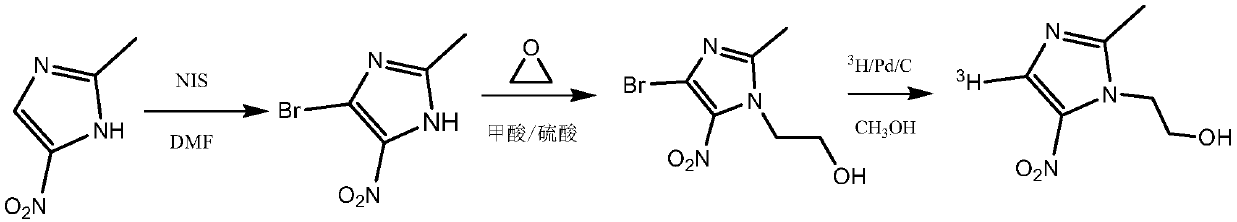
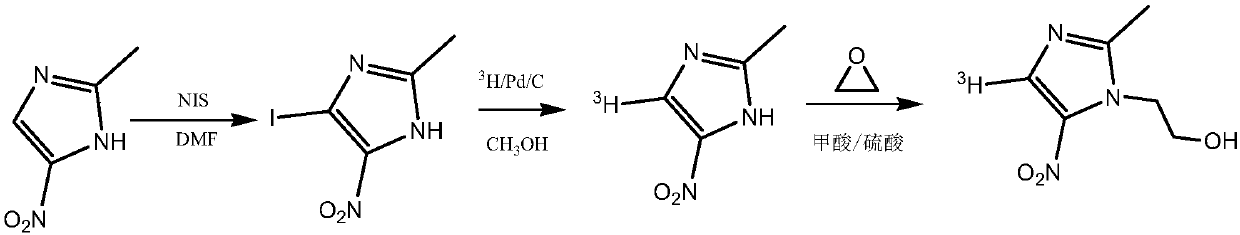
Tritiated metronidazole and preparation method thereof
InactiveCN106674123ARaw materials are easy to getReduce pollutionOrganic chemistry methodsNitroimidazoleRadioactive tracer
The invention belongs to the field of radioactive isotope labeling preparation, and particularly relates to tritiated metronidazole and a preparation method thereof. The preparation method includes: using 2-methyl-5-nitroimidazole as a raw material to react with N-iodosuccinimide to obtain 4-iodine-2-methyl-5nitroimidazole; under catalysis of palladium carbon, enabling 4-iodine-2-methyl-5nitroimidazole and tritium gas to be in tritium-halogen exchange to generate 4-3H-2-methyl-5-nitroimidazole; enabling 4-3H-2-methyl-5-nitroimidazole to react with ethylene oxide to obtain 4-3H-metronidazole. A synthetic product is purified through a prepared liquid phase to obtain4-3H-metronidazole with high specific activity (22.08Ci / g), high radiochemical purity (greater than or equal to 98%) and high chemical purity (greater than or equal to 98%). The tritiated metronidazole can be used as a radioactive tracer in studying absorption, distribution, metabolism and residue elimination of metronidazole in animal bodies.
Owner:HUAZHONG AGRI UNIV
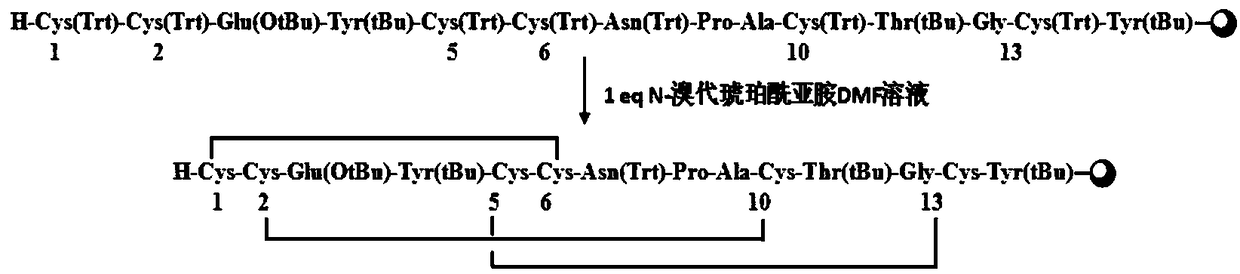
A synthetic method for linaclotide
ActiveCN109311941AAvoid repeated foldingAvoid it happening againPeptide preparation methodsBulk chemical productionN-BromosuccinimideAmino acid side chain
The invention relates to the field of pharmaceutical synthesis, and discloses a synthetic method for linaclotide. The method uses a solid phase one-step cyclization method to prepare linaclotide, andthe linaclotide linear peptide resin is directly cyclized by a N-X-substituted succinimide solution oxidation system without cleavage to obtain linaclotide resin, the resin is cleaved, purified and lyophilized to give linaclotide. The N-X-substitured succinimide is one of N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide, and N-hydroxy thiosuccinimide. The method has the following advantages that: 1) solid phase cyclization is adopted, firstly, the pseudo-dilution effect is achieved, repeated folding of the peptide chain is avoided, and the cyclization reaction can be carried out at ahigher concentration, which can greatly improve the production efficiency; secondly, the linear peptide resin is not cleaved before cyclization, avoiding the production of a large amount of impurities and improving the efficiency of linaclotide cyclization; 2) one-step cyclization using N-X-substituted succinimide can avoid multi-step purification of the intermediates, reduce the composition of the intermediate purification step, and improve the total yield of linaclotide; and 3) a specific amino acid side chain protecting group is adopted, thus positioning a pair of disulfide bonds in the cyclization process, reducing the formation of mismatch by-products, improving the purity of linaclotide, greatly improving production efficiency, and reducing the manufacturing cost.
Owner:SHENZHEN JYMED TECH
Method for stereoselective preparation of derivatives of pyrane
InactiveCN102180923AReduce pollutionEasy to operateBiocideSugar derivativesReaction temperatureSolvent
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and in particular relates to a method for stereoselective preparation of derivatives of pyrane. The method comprises the following steps of: under the protection of inert gases, adding derivatives of 1-p-tolylthio-2-C-glyoxyl-alpha-D-glucopyranose, derivatives of 1-thiophenyl-2-C- glyoxyl-alpha-D-glucopyranose or derivatives of 1-ethylthio p-tolyl-2-C-glyoxyl-alpha-D-glucopyranose, which serve as reactants, into a reactor, adding alcohol, phenol, trimethylsilyl azide or monosaccharide derivatives, adding a solvent such as methylene chloride to dissolve the raw materials, and performing the reaction at the reaction temperature of between 40 DEG C below zero and room temperature in the presence of a catalyst which is N-iodosuccinimide, or N-bromosuccinimide, or copper bromide and tetrabutylammonium bromide, or iodine, bromine simple substance or N-iodosuccinimide and trimethylsilyl triflate, or trifluoromethanesulfonic silver, or paratoluenesulfonic acid or trifluoromethanesulfonic acid to obtain the derivatives of pyrane. The method has the advantages of simple operation, high selectivity, low cost, light environmental pollution and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Process for synthesizing optical activity 2-(1'(Z) alkenyl iodo-alkyl) tetrahydrofuran
InactiveCN101440076AEasy to separate and purifyShort reaction timeAsymmetric synthesesIodidePhotochemistry
The invention discloses a method for synthesizing optically active (S)-2-(1'(Z)alkenyl iodoalkyl)tetrahydrofuran. An optically active (R)-4, 5-allenol is taken as a reaction substrate and is subjected to iodocyclization reaction with an electrophilic reagent, namely N-iodosuccinimide to generate the optically active (S)-2-(1'(Z)alkenyl iodoalkyl)tetrahydrofuran (large amount) and (R)-2-(1'(E)alkenyl iodoalkyl)tetrahydrofuran (small amount); and the optically active (S)-2-(1'(Z) alkenyl iodoalkyl)tetrahydrofuran with Z configuration is obtained by utilizing the speed difference that a Z / E isomer is subjected to Sonogashira coupling reaction with propiolic alcohol under the co-catalysis of dichlorobis(triphenylphosphine)palladium and cuprous iodide. The method has simple operation, is easy to obtain the raw material and the reagent, has the reaction with high region and stereoselectivity, can synchronously introduce a plurality of substitional groups, is easy to separate and purify a product, and is suitable to synthesize various substituted optically active tetrahydrofuran compounds.
Owner:ZHEJIANG UNIV
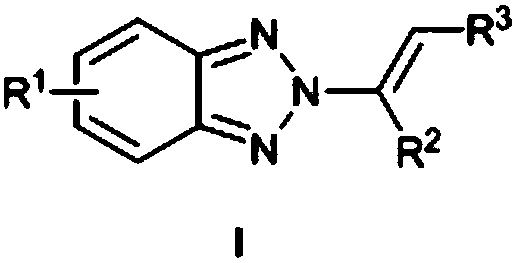
N2-alkenyl benzotriazole derivative and synthetic method thereof
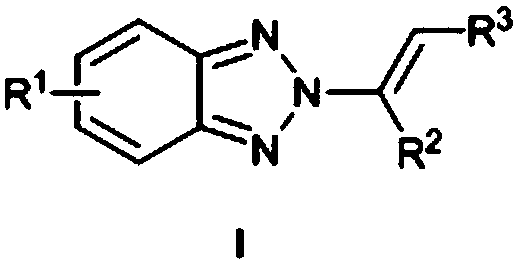
ActiveCN109134392ANo participationSimple and mild reaction conditionsOrganic chemistryRoom temperatureBenzotriazole
The invention provides a N2-alkenyl benzotriazole derivative which is a compound as shown in a formula. A synthetic method comprises the following steps: carrying out a reaction among benzotriazole, N-iodosuccinimide and olefin at a room temperature for 0.5-5 hours by taking chloroform as a solvent and M(OTf)n as a catalyst; then carrying out condensation; carrying out a reaction at 50-70 DEG C for 8-15 hours by taking methanol as a solvent to obtain the target product. The method has the obvious characteristic that the reaction conditions are simple and mild, no transitional metals participate in the reaction and the yield and N2-selectivity are relatively high. At present, no method of constructing N2-alkenyl benzotriazole directly by means of a one-pot method is reported, and the synthetic method is quite simple and cheap and simple to operate, achieves the N2-selectivity which is over 84%, and is high in reaction universality.
Owner:ZHOUKOU NORMAL UNIV +1
Synthesis method of eribulin intermediate
ActiveCN107973804AEasy to operateHigh purityGroup 4/14 element organic compoundsBulk chemical productionState of artEribulin
The invention discloses a synthesis method of an eribulin intermediate. The synthesis method mainly comprises the following steps: taking a compound shown as a formula 1 as a raw material to react with N-iodosuccinimide and then carrying out hydroxyl protection reaction to obtain a target product, namely a compound shown as a formula 4. The synthesis method provided by the technical scheme has theadvantages of simple and convenient operation steps, moderate reaction conditions and a few of byproducts in a reaction process; compared with the prior art, the productivity and the yield of the target product are remarkably improved and the synthesis method is very suitable for industrial production.
Owner:WUYAN PHARM TECH (SHANGHAI) CO LTD
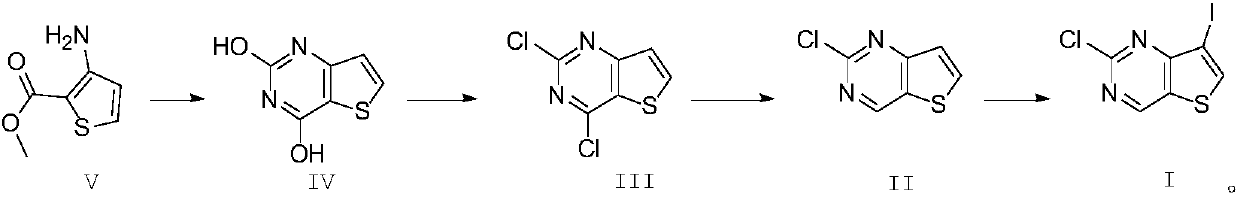
Preparation method of 2-chlorine-7-iodothieno[3,2-D] pyrimidine
ActiveCN102924473AReduce manufacturing costSimple reaction conditionsOrganic chemistryEthyl ChlorideChemistry
The invention discloses a preparation method of 2-chlorine-7-iodothieno[3,2-D] pyrimidine. 3-amino-2-thiophene methyl formate, urea, phosphorus oxychloride, N-iodosuccinimide (NIS) and the like serve as the raw materials, and under the action of 10wt% of a palladium-charcoal catalyst, a target product 2-chlorine-7-iodothieno[3,2-D] pyrimidine is obtained after four steps of reactions. The method is simple and convenient to operate, mild in reaction conditions, environmental-friendly and suitable for industrialization scale production; the total yield is higher than 75% and is greatly improved compared with the existing yield of 35%; and the production cost of the existing drugs is reduced remarkably.
Owner:上海毕得医药科技股份有限公司
Method for preparing hydroxymethyl furfural by catalytic dehydration reaction of hexose
InactiveCN102212048ALow priceEasy to getOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsN-ChlorosuccinimideSolvent
The invention provides a method for preparing hydroxymethyl furfural by catalytic dehydration reaction of hexose. The method comprises the following steps of: dissolving reaction substrates of hexose into a solvent, and adding a catalyst of halogenated succinimide to form a reaction system; and at the temperature of between 0 and 200 DEG C and under the protection of atmosphere gas, stirring the reaction system by magnetic force for dehydration reaction for 0.5 to 10 hours to prepare the hydroxymethyl furfural, wherein the catalyst of the halogenated succinimide may be N-bromosuccinimide, N-chlorosuccinimide, N-iodosuccinimide, N-fluorosuccinimide or any succinimide derivatives with substituted halogen atoms. The method has the advantages that: the catalyst of the halogenated succinimide is low in price and easily bought, and can catalyze the hexose in high efficiency as well as high selectivity to prepare the hydroxymethyl furfural; the method is environment-friendly, and the productis easily disposed; and in the whole process, only regenerated compounds such as fructose are consumed, the cost is low, the technically economical requirement is met, and the application prospect isbroad.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
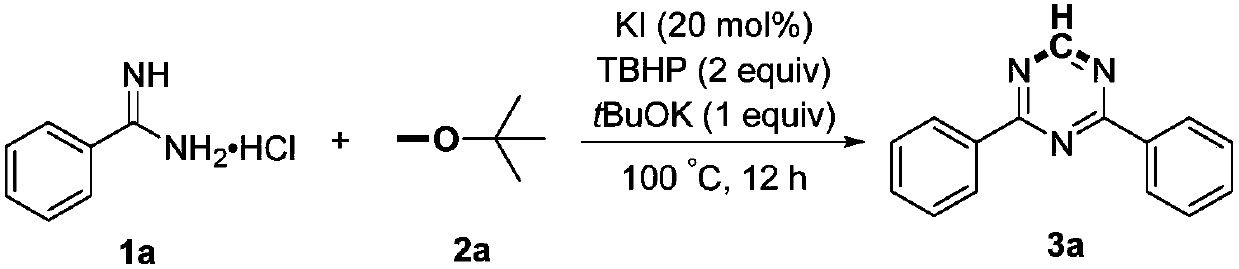
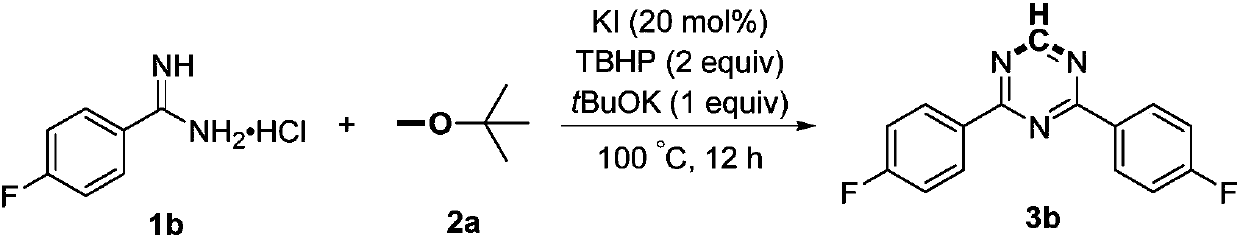
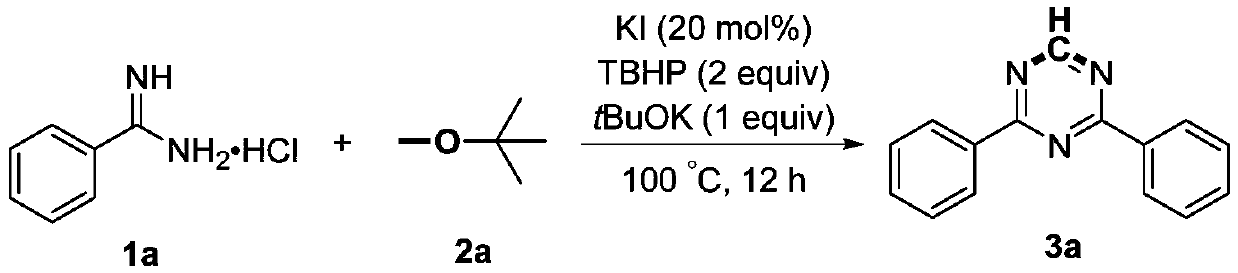
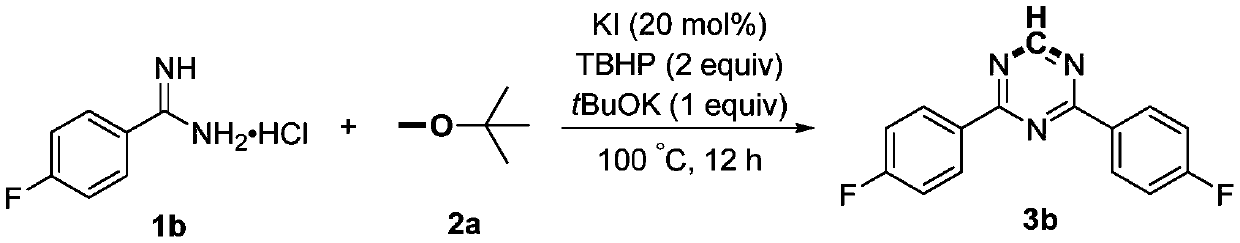
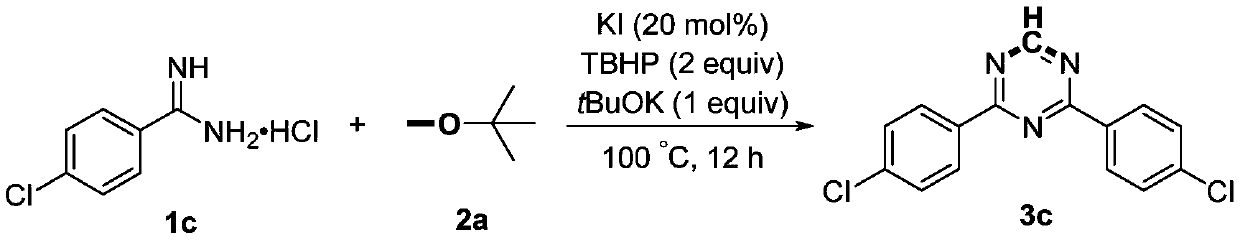
Preparation method of 2,4-di-substituted-1,3,5-triazine
ActiveCN107759530AWide variety of sourcesEasy to getOrganic chemistryPotassium iodinePotassium carbonate
The invention discloses a method for preparing 2,4-di-substituted-1,3,5-triazine. The method comprises the specific step: with substituted formamidine hydrochloride as a reaction substrate an iodine-containing compound as a catalyst, tert-butyl hydroperoxide as an oxidant, inorganic base as an acid binding agent and aliphatic ether as an organic solvent (also used as a carbon source), carrying outcarbon-hydrogen and carbon-oxygen bond deletion, nucleophilic addition, deaminizing condensation and oxidative aromatization reaction to obtain a 2,4-di-substituted-1,3,5-triazine compound, wherein achemical structural general formula of the substituted formamidine hydrochloride is shown in the description; the iodine-containing compound is selected from one of potassium iodide (KI), tetrabutylammonium iodide (TBAI), elemental iodine (I2) and N-iodosuccinimide (NIS); the inorganic base is selected from one of anhydrous potassium carbonate, anhydrous sodium carbonate, cesium carbonate, potassium hydroxide and potassium tert-butoxide; and the aliphatic ether is selected from one of methyl tert-butyl ether, ethyl ether and ethylene glycol dimethyl ether. The preparation method disclosed bythe invention has the characteristics of easily-obtained raw materials, low price and low toxicity of a catalyst, wide range of the substrate, simplicity and convenience in operation, greenness, environmental protection and the like.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
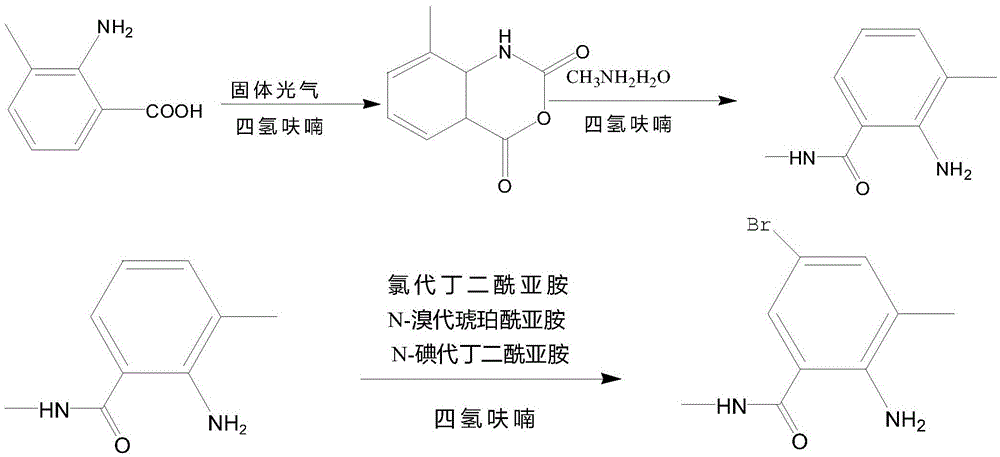
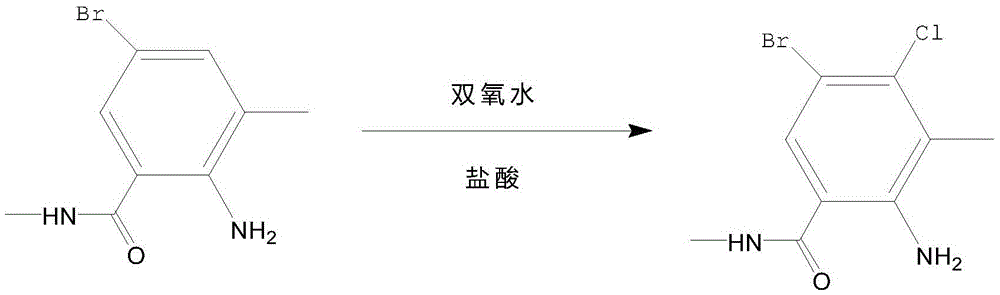
Synthetic method of 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide
InactiveCN105523951AOrganic compound preparationCarboxylic acid amides preparationN-BromosuccinimideChemical synthesis
The invention relates to a synthetic method of 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide and belongs to the field of chemical synthesis. The method comprises the following steps: using tetrahydrofuran and tetrahydrofuran as raw materials to react under phosgene catalytic action, adjusting pH by dropwise adding a sodium bicarbonate solution, simultaneously carrying out a reaction between chlorosuccinimide, N-iodosuccinimide and N-bromosuccinimide, carrying out suction filtration, and oxidizing so as to prepare 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide. The method has advantages of simple operation and high synthesis rate.
Owner:CHANGZHOU UNIV
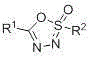
Oxathiadiazole-2-oxide compound and preparation method thereof
The invention relates to an oxathiadiazole-2-oxide compound and a preparation method thereof. The structural formula of the prepared oxathiadiazole-2-oxide compound is shown in a drawing, wherein R<1> and R<2> are hydrogen atoms, alkyl groups, aryl groups and the like. The preparation method comprises the following steps: sulfonylhydrazone, an additive and a base react at the temperature of 0-25 DEG C for 1-24 h; extraction, washing in an organic solvent and concentration are performed after reaction, column chromatographic purification is performed, the 1,2,3,4-oxathiadiazole-2-oxide compound is obtained, and the yield is 60%-95%, wherein the additive is N-iodosuccinimide, N-chlorosuccinimide, N-bromosuccinimide, iodine or other additives; the base is sodium carbonate, potassium carbonate, cesium carbonate, potassium hydroxide, sodium methoxide or other bases.
Owner:HARBIN INST OF TECH AT WEIHAI
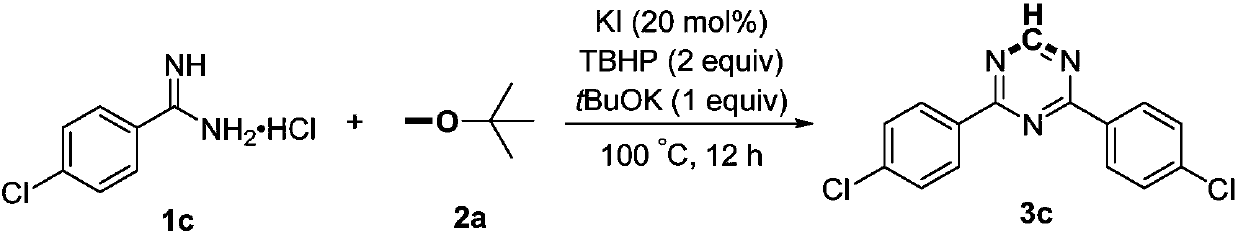
Process for preparing 3-iodine-6-(trifluoromethyl) imidazo [1,2-a] pyridine
InactiveCN107118210ARaw materials are cheap and easy to getOperating conditions are mild and easy to controlOrganic chemistryIodineAcetal
The invention discloses a process for preparing 3-iodine-6-(trifluoromethyl) imidazo [1,2-a] pyridine. The process comprises the following steps: by taking 2-amino-5-trifluoromethyl picoline as a raw material, performing reaction with bromoacetaldehyde diethyl acetal to generate 6-(trifluoromethyl) imidazo [1,2-a] pyridine, and performing reaction on the 6-(trifluoromethyl) imidazo [1,2-a] pyridine with N-iodosuccinimide, thereby obtaining a target compound, namely 3-iodine-6-(trifluoromethyl) imidazo [1,2-a] pyridine. The process is low in price of raw materials, easy in raw material obtaining, relatively short in reaction time and beneficial to industrial application.
Owner:GUIZHOU UNIV
A kind of tritium-labeled metronidazole and preparation method thereof
InactiveCN106674123BRaw materials are easy to getReduce pollutionOrganic chemistry methodsNitroimidazoleRadioactive tracer
The invention belongs to the field of radioactive isotope labeling preparation, and particularly relates to tritiated metronidazole and a preparation method thereof. The preparation method includes: using 2-methyl-5-nitroimidazole as a raw material to react with N-iodosuccinimide to obtain 4-iodine-2-methyl-5nitroimidazole; under catalysis of palladium carbon, enabling 4-iodine-2-methyl-5nitroimidazole and tritium gas to be in tritium-halogen exchange to generate 4-3H-2-methyl-5-nitroimidazole; enabling 4-3H-2-methyl-5-nitroimidazole to react with ethylene oxide to obtain 4-3H-metronidazole. A synthetic product is purified through a prepared liquid phase to obtain4-3H-metronidazole with high specific activity (22.08Ci / g), high radiochemical purity (greater than or equal to 98%) and high chemical purity (greater than or equal to 98%). The tritiated metronidazole can be used as a radioactive tracer in studying absorption, distribution, metabolism and residue elimination of metronidazole in animal bodies.
Owner:HUAZHONG AGRI UNIV
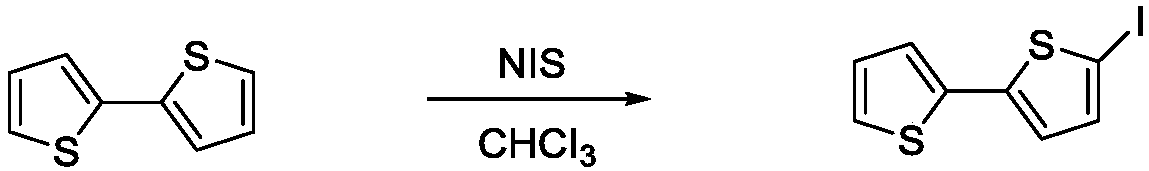
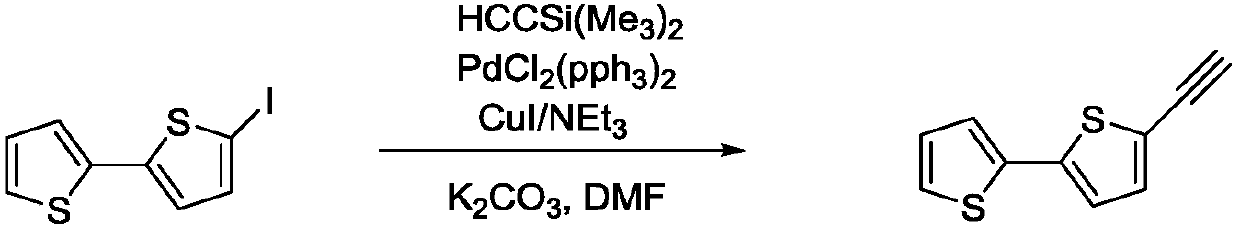
Extended type fluorescent nucleoside analog and preparing method thereof
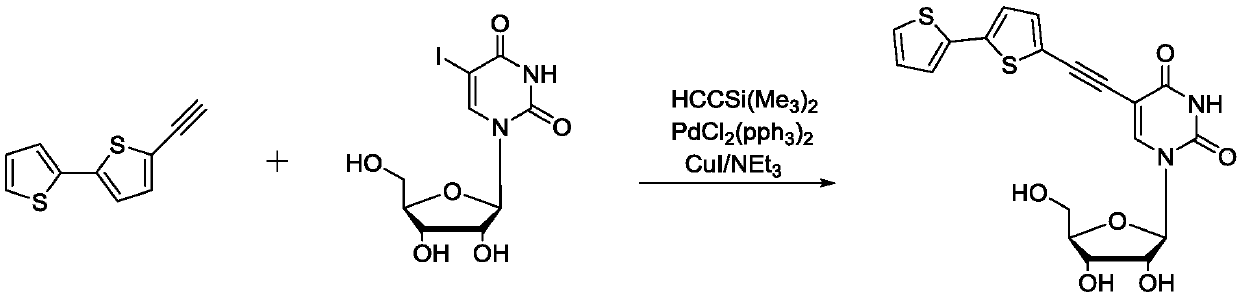
InactiveCN106699827AExcellent fluorescence propertiesSugar derivativesSugar derivatives preparationN dimethylformamideFluorescence
The invention aims at providing a synthesis method of obtaining a fluorescent nucleoside analog by adopting 2, 2'-bithiophene and 5-iodouridine as raw materials and through a reaction with a palladium catalyst. The method is characterized by comprising the steps of 1, stirring 2, 2'-bithiophene and N-iodosuccinimide for seven hours at the temperature of 20-50 DEG C, and obtaining an intermediate product through treatment; 2, stirring the product in the last step and the catalyst for 4-6 hours at the temperature of 50-70 DEG C, reducing the temperature to the room temperature, adding a certain amount of potash, stirring for 3 hours again, and obtaining 2, 2'-bithiophene acetylide; 3, dissolving the product in the last step, 5-iodouridine, a certain amount of palladium catalyst and cuprous iodide into N, N-dimethylformamide to be subjected to a reaction for 2-5 hours at the temperature of 65-70 DEG C, and obtaining a final product through separation and purification. The fluorescent nucleoside analog is similar to natural nucleoside in structure, has good fluorescence characteristics, can be applied as a fluorescent nucleoside probe, and has a good application prospect in the fields of biochemistry, medicine and the like.
Owner:JIANGNAN UNIV
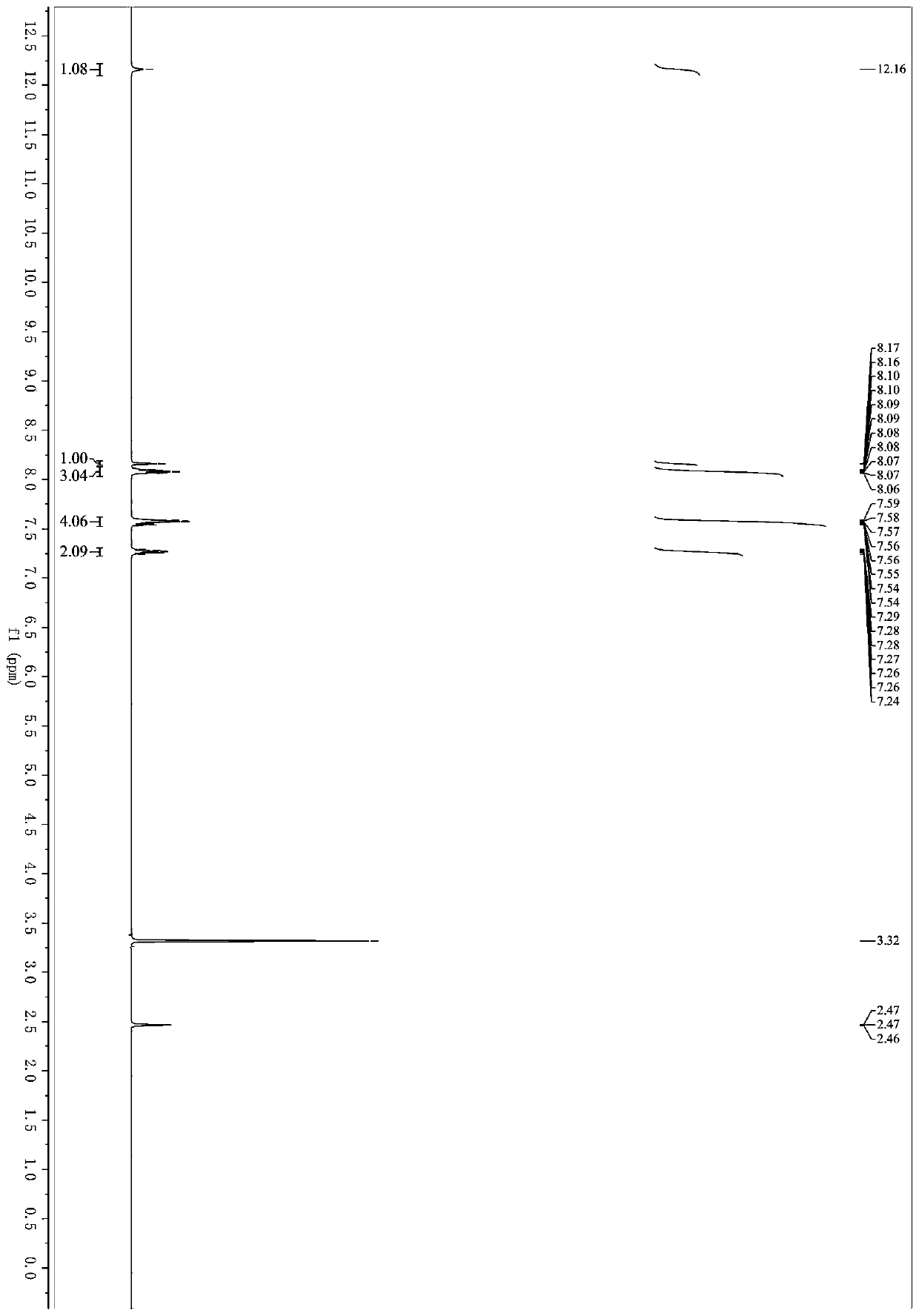
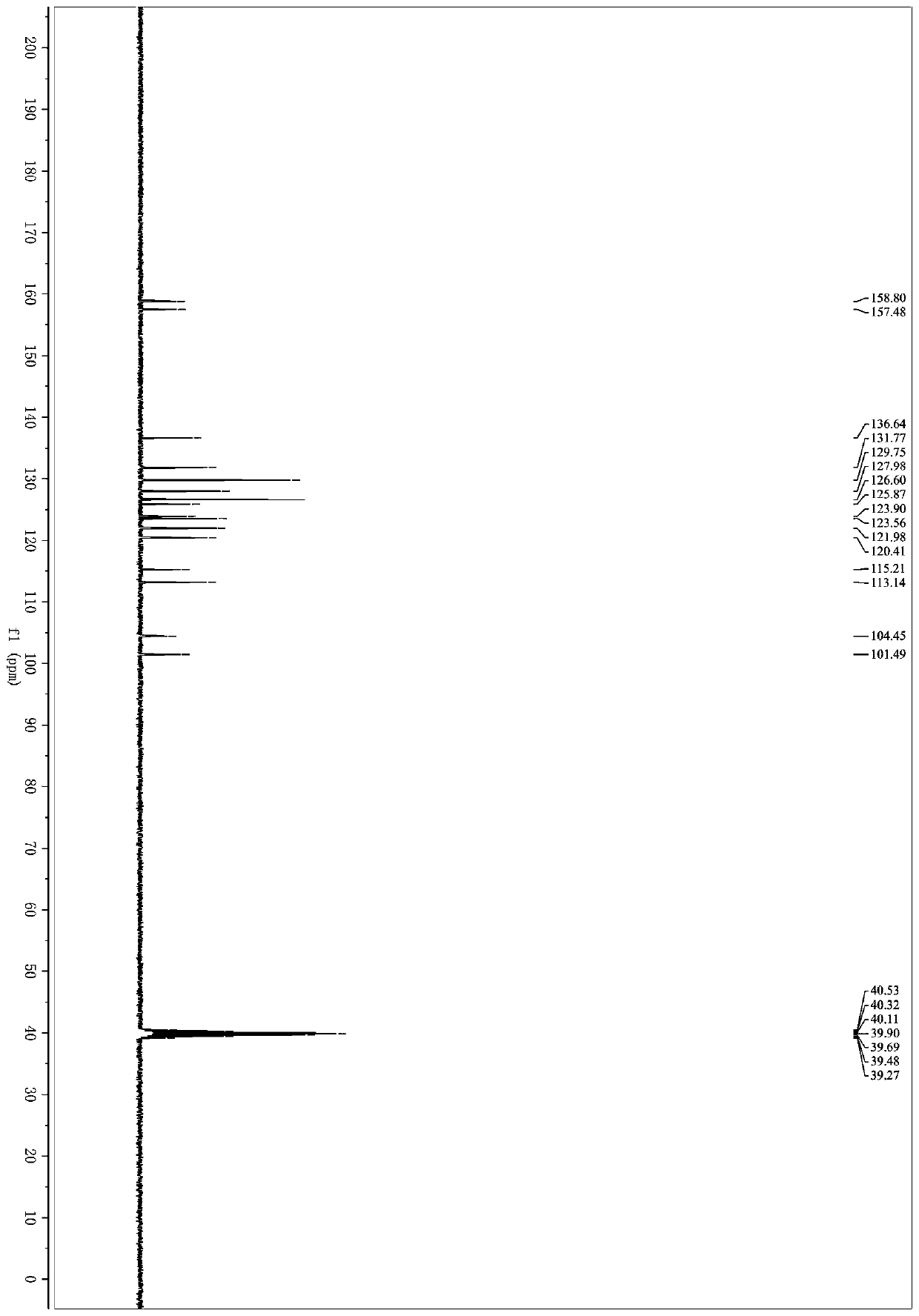
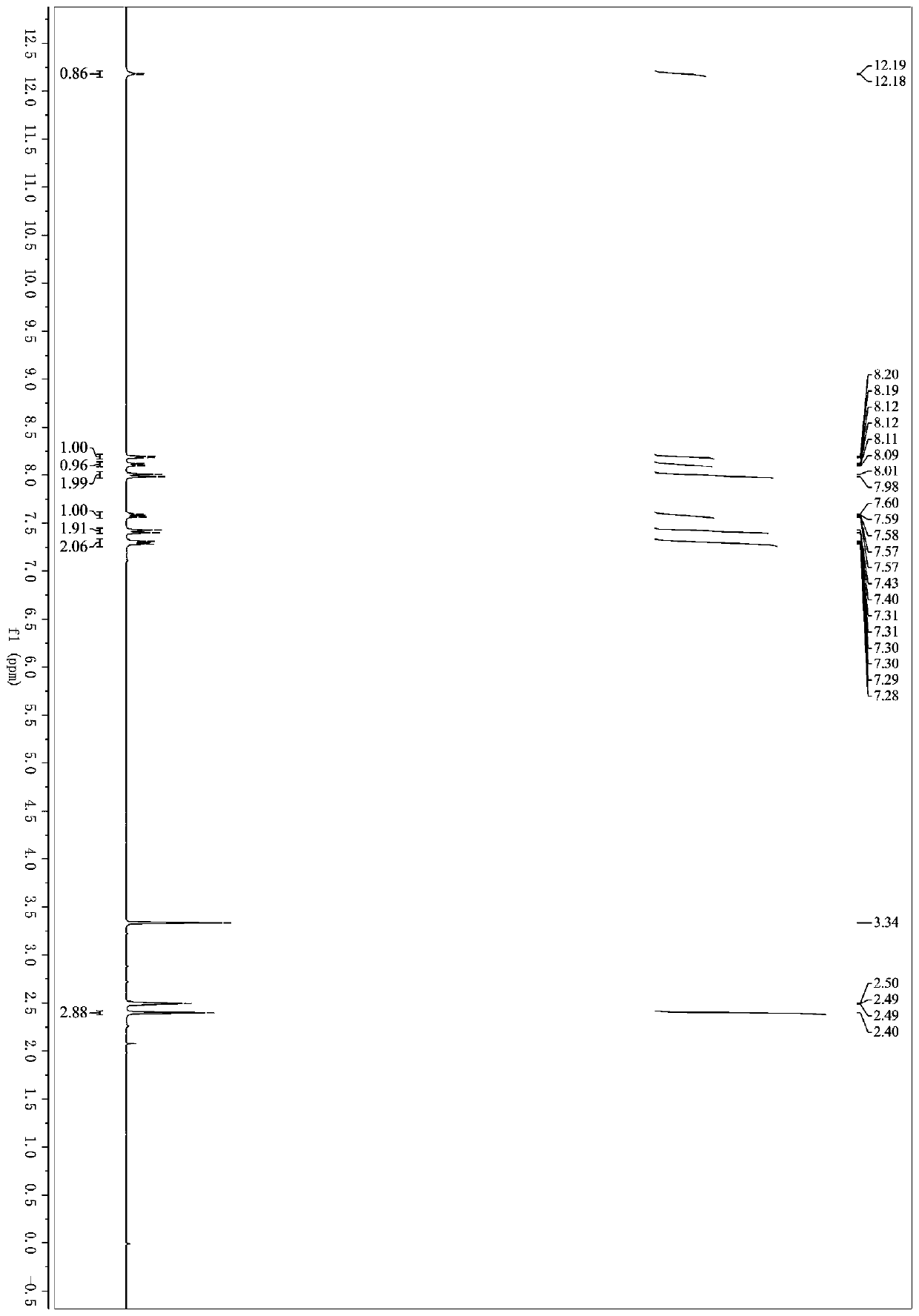
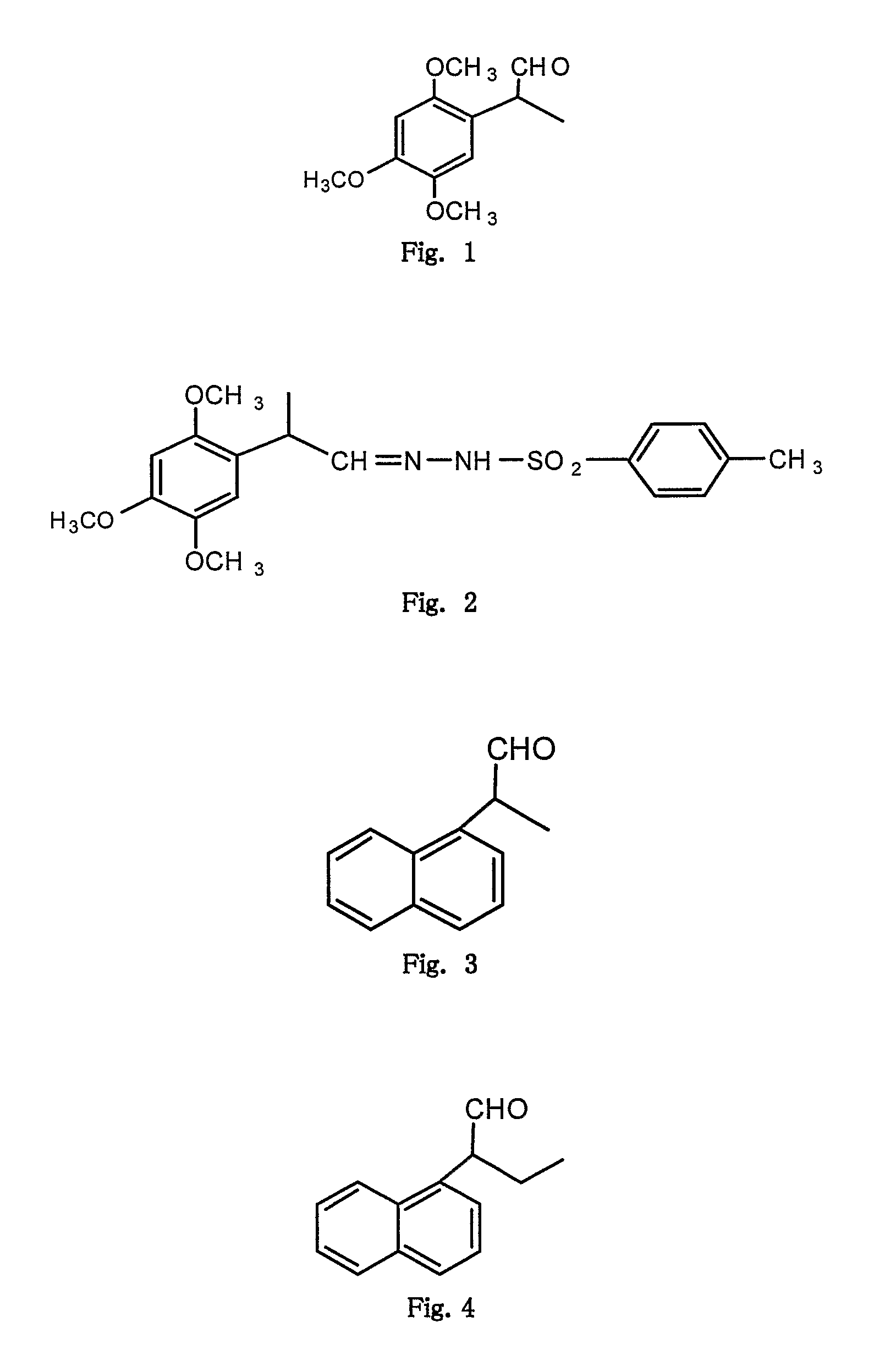
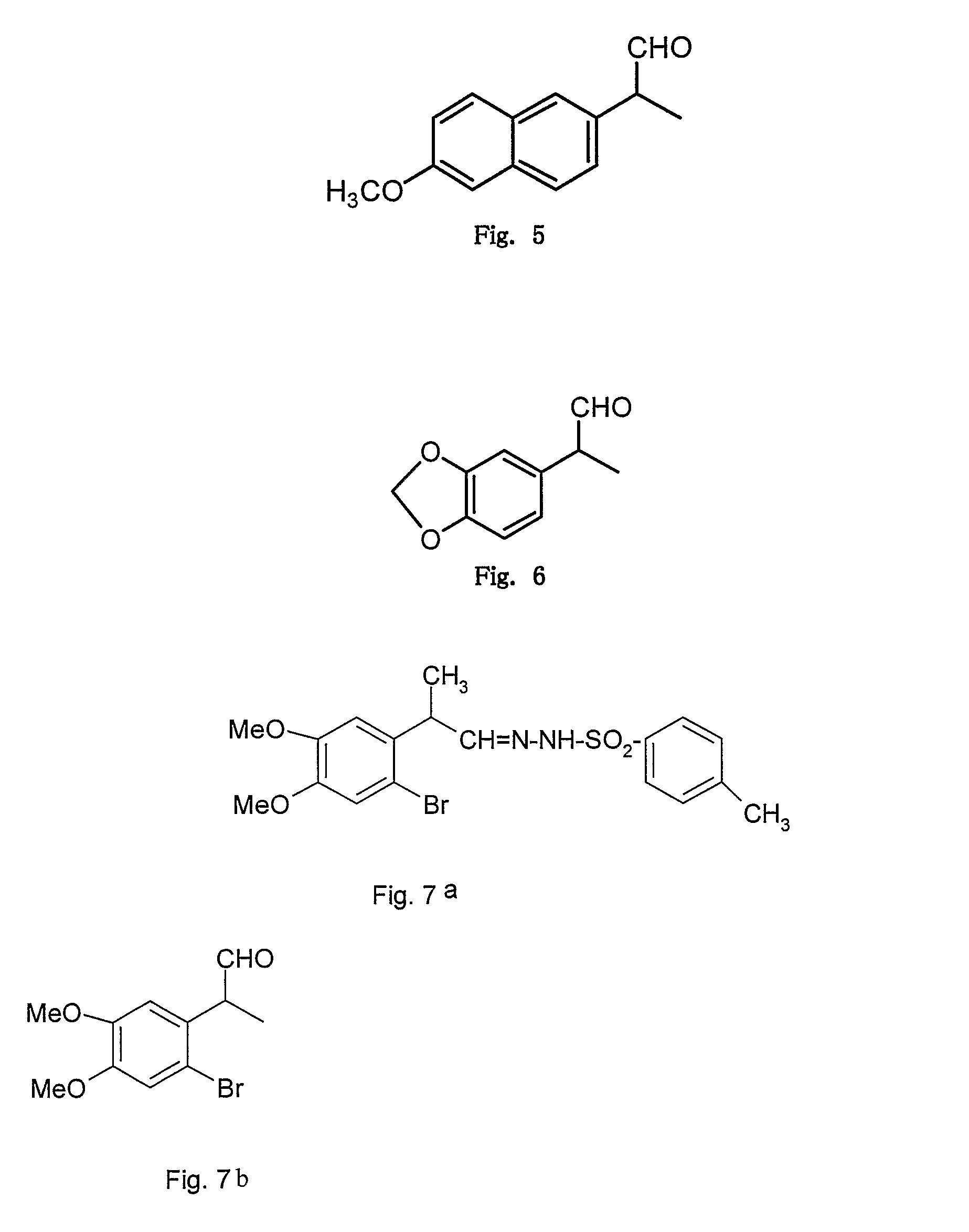
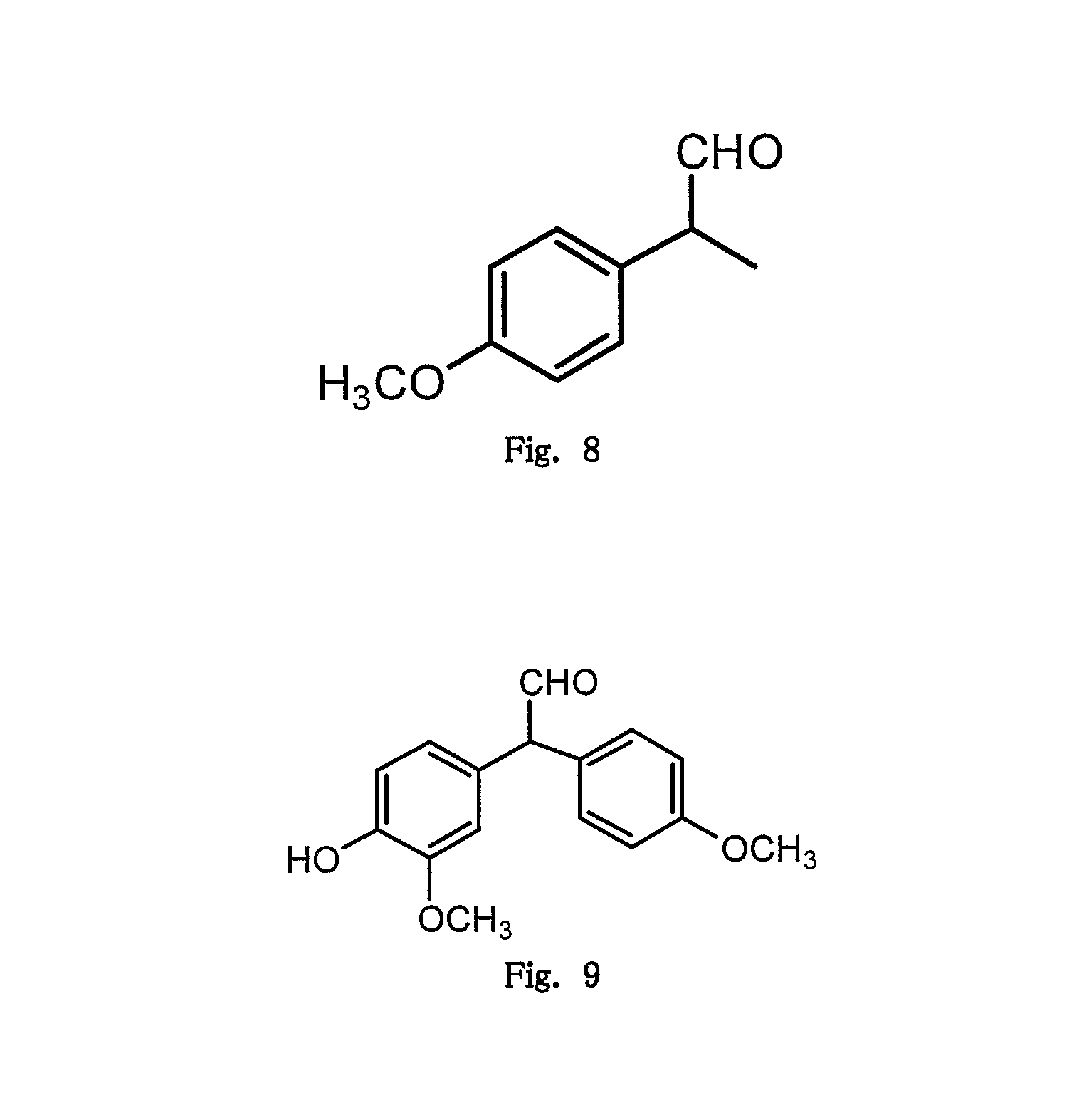
Microwave induced single step green synthesis of some novel 2-aryl aldehydes and their analogues
InactiveUS20120041234A1Potent anticancer activityOrganic compound preparationCarbonyl compound preparation by oxidationNonsteroidal Antiinflammatory Drugs/NSAIDsPhenstatin
The present invention provides a process for the preparation of some novel 2-aryl and 2,2-diaryl aldehydes and analogues which are privileged intermediates for commercially important nonsteroidal anti-inflammatory drugs including naproxen, flurbiprofen and potent anticancer drug candidates, including phenstatin through a unique single step synthetic methodology utilizing easily available substrates in the form of aryl alkenes as well as environmentally benign aqueous reaction conditions in the form of solvents such as mixtures of water and DMSO or Dioxane and reagents N-bromosuccinimide, N-iodosuccinimide, N-cholorosuccinimide and phase transfer catalyst such as cetyltrimethyl ammonium bromide, N-hexyl ammonium chloride for a reaction time varying from 1 min-30 min, depending upon microwave or conventional heating, without using expensive transition metal catalysts or lewis acids / bases with yield varying from 35-55%, depending upon the solvent and substrate used. The developed method provides a clean and convenient alternative to access a diverse range of medicinally important 2-aryl and 2,2-diaryl aldehyde based scaffolds in lieu of the conventional multistep protocols employing expensive and hazardous transition metal catalysts and lewis acids / bases.
Owner:COUNCIL OF SCI & IND RES
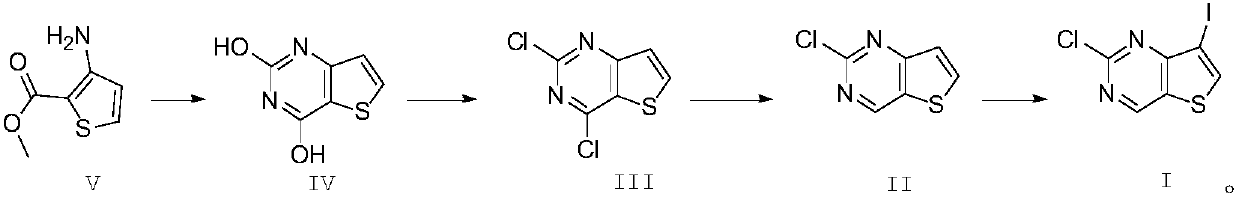
Synthesis method of JAK kinase inhibitor intermediate
The invention relates to a preparation method of 2-chlorine-7-iodothieno[3,2-D]pyrimidine. The 2-chlorine-7-iodothieno[3,2-D]pyrimidine is prepared from 3-amino-2-methyl thiophenecarboxylate serving as a starting material through urea cyclization, chlorination, reduction and iodination. The preparation method is simple and convenient in operation and mild in reaction condition, in particular, N-iodosuccinimide is generated by in-situ reaction to perform iodination, so that environmental friendliness is achieved and the yield of the iodination reaction is greatly increased; meanwhile, the production cost is reduced, and the preparation method is obviously improved compared with the prior art and is suitable for industrialized large-scale production.
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
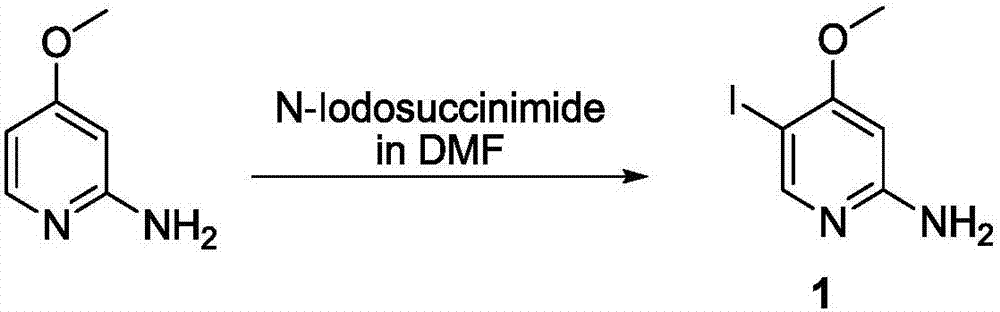
Method for synthesizing 5-iodo-4-methoxy-2-aminopyridine
InactiveCN107011256AMild reaction conditionsThe synthetic route is simpleOrganic chemistry4-methoxypyridineReagent
The invention provides a method for synthesizing 5-iodo-4-methoxy-2-aminopyridine. The method comprises the following steps: carrying out a reaction on 2-amino-4-methoxypyridine used as an initial material by using N-iodosuccinimide as an iodization reagent, and carrying out post-treatment and purification after the reaction is finished in order to obtain the 5-iodo-4-methoxy-2-aminopyridine. Compared with the prior art, the method provided by the invention has the advantages of mild reaction conditions, simple synthesis route, short reaction time and low cost.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Preparation and medical application of binary structure sinomenine derivatives
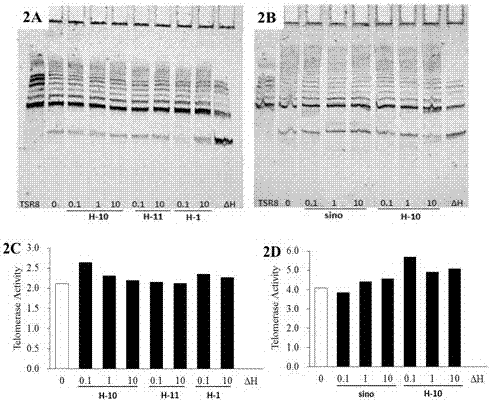
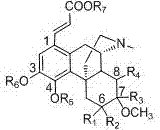
The invention relates to preparation and a medical purpose of a dual structure sinomenine derivative, in particular relates to synthesis of the dual structure sinomenine derivative and application of the dual structure sinomenine derivative in preparing anti-inflammatory and neurodegenerative disease resistant medicines and belongs to the technical field of medicines. The dual structure sinomenine derivative is prepared by the following steps: with sinomenine or a sinomenine structural analog as a raw material, carrying out reaction on the sinomenine or the sinomenine structural analog and N-iodosuccinimide (NIS) to prepare 1-iodo sinomenine or sinomenine structural analog; and further, by virtue of Heck reaction, carrying out coupling on the 1-iodo sinomenine or sinomenine structural analog and acrylate to obtain a 1-acrylate substituted dual sinomenine derivative. The dual structure sinomenine derivative is adopted to carry out enzyme, cell and mouse drug effect tests, and a result shows that the dual structure sinomenine derivative is capable of inhibiting NF-kappa B expression, resisting inflammation, protecting PC12 neurons, lowering the quantity of NO, released by microglia BV-2, and strengthening activities including telomerase and has good potential of resisting inflammation and neurodegenerative diseases and clinical application value.
Owner:JIANGSU UNIV
A kind of preparation method of 2,4-disubstituted-1,3,5-triazine
ActiveCN107759530BWide variety of sourcesEasy to getOrganic chemistryPotassium iodinePotassium carbonate
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Method for stereoselective preparation of derivatives of pyrane
InactiveCN102180923BReduce pollutionEasy to operateBiocideSugar derivativesReaction temperaturePyran
The invention belongs to the technical field of organic chemistry and pharmaceutical chemistry, and in particular relates to a method for stereoselective preparation of derivatives of pyrane. The method comprises the following steps of: under the protection of inert gases, adding derivatives of 1-p-tolylthio-2-C-glyoxyl-alpha-D-glucopyranose, derivatives of 1-thiophenyl-2-C- glyoxyl-alpha-D-glucopyranose or derivatives of 1-ethylthio p-tolyl-2-C-glyoxyl-alpha-D-glucopyranose, which serve as reactants, into a reactor, adding alcohol, phenol, trimethylsilyl azide or monosaccharide derivatives, adding a solvent such as methylene chloride to dissolve the raw materials, and performing the reaction at the reaction temperature of between 40 DEG C below zero and room temperature in the presence of a catalyst which is N-iodosuccinimide, or N-bromosuccinimide, or copper bromide and tetrabutylammonium bromide, or iodine, bromine simple substance or N-iodosuccinimide and trimethylsilyl triflate, or trifluoromethanesulfonic silver, or paratoluenesulfonic acid or trifluoromethanesulfonic acid to obtain the derivatives of pyrane. The method has the advantages of simple operation, high selectivity, low cost, light environmental pollution and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
4-cyano-5-(3-indolyl)oxazole compounds and preparation method thereof
Provided are a 4-cyano-5-(3-indolyl) oxazole compound and a preparation method thereof. 3- (1H-indole-3-group)-3-oxy propionitrile and benzylamine are used as raw materials, and 5-(3-indole) oxazole is a heterocyclic skeleton. The formula is as shown in the description, wherein R1 is hydrogen, methyl, ethyl, tertiary butyl, tertiary butyl oxy carbonyl or benzyl, R2 is halogen, ester, carboxyl, cyano, amino or methoxy; benzylamine Ar is alkyl or alkoxy-substituted aromatic hydrocarbon, halogenated aromatics or heteroaromatic aromatics, iodisan is one of iodine, potassium iodide, sodium iodide,tetrabutyl ammonium iodide, sulfonium iodate salt, cesium iodide and N-Iodosuccinimide, oxidizing agent is one of hydrogen peroxide, peroxy tert butyl alcohol, potassium hydrogen persulfate, ditert butyl peroxide and chloramine salt, and solvent is one or two of ethyl acetate, acetonitrile, N, N-dimethylformamide, diethyl ether, dimethyl sulfoxide, 1,2-dichloroethane, methyl alcohol, ethyl alcohol, isopropanol, n-butyl ether, n-butyl alcohol, benzene, methylbenzene, 1,4-dioxane, methyl tert-butyl ether, dichloromethane, cyclohexane, petroleum ether, carbon tetrachloride, trichloromethane and tetrahydrofuran.
Owner:ZUNYI MEDICAL UNIVERSITY
Microwave induced single step green synthesis of some novel 2-aryl aldehydes and their analogues
InactiveUS8779200B2Potent anticancer activityOrganic compound preparationCarbonyl compound preparation by oxidationNonsteroidal Antiinflammatory Drugs/NSAIDsPhenstatin
The present invention provides a process for the preparation of some novel 2-aryl and 2,2-diaryl aldehydes and analogues which are privileged intermediates for commercially important nonsteroidal anti-inflammatory drugs including naproxen, flurbiprofen and potent anticancer drug candidates, including phenstatin through a unique single step synthetic methodology utilizing easily available substrates in the form of aryl alkenes as well as environmentally benign aqueous reaction conditions in the form of solvents such as mixtures of water and DMSO or Dioxane and reagents N-bromosuccinimide, N-iodosuccinimide, N-cholorosuccinimide and phase transfer catalyst such as cetyltrimethyl ammonium bromide, N-hexyl ammonium chloride for a reaction time varying from 1 min-30 min, depending upon microwave or conventional heating, without using expensive transition metal catalysts or lewis acids / bases with yield varying from 35-55%, depending upon the solvent and substrate used. The developed method provides a clean and convenient alternative to access a diverse range of medicinally important 2-aryl and 2,2-diaryl aldehyde based scaffolds in lieu of the conventional multistep protocols employing expensive and hazardous transition metal catalysts and lewis acids / bases.
Owner:COUNCIL OF SCI & IND RES
Synthesis method of 2-amino-5-iodopyrazine
InactiveCN106674137AReasonable priceMild reaction conditionsOrganic chemistryOrganic synthesisSynthesis methods
The invention belongs to the field of organic synthesis, and particularly relates to a synthesis method of 2-amino-5-iodopyrazine. The synthesis method of 2-amino-5-iodopyrazine comprises the following steps: carrying out a reaction on 2-aminopyrazine and N-iodosuccinimide which serve as raw materials under the action of a proper solvent at proper temperature for hours to generate 2-amino-5-iodopyrazine, and carrying out purification to obtain pure 2-amino-5-iodopyrazine. The synthesis method of 2-amino-5-iodopyrazine has the beneficial effects that the reaction raw materials are readily available and reasonable in price; the reaction conditions are mild; the synthesis method is easy to operate and control; post-treatment is simple; the product quality is stable; and the purity is high.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Method for synthesizing (2-iodine-2-aryl) ethyl aryl ether derivatives
InactiveCN110015981APromote incomeGood group toleranceSulfide preparationChemical industryOrganic synthesis
The invention discloses a method for synthesizing (2-iodine-2-aryl) ethyl aryl ether derivatives, belonging to the technical field of organic synthesis intermediates. The method comprises the following steps: 1) adding styrene derivative, diaryl disulfide, N-iodosuccinimide and 1,2-dichloroethane, tightening the bottle stopper of a reaction tube, and magnetically stirring for 1 hour at 80 DEG C; 2) after that reaction, extracting ethyl acetate, combining the organic phases, distilling under reduced pressure to remove most of the solvent to obtain crude product, and carrying out column chromatography separation and purification on the crude product with petroleum ether and ethyl acetate in a volume ratio of 5:1-30:1 as eluent to obtain the target product. The method has wide application inmedicine, pesticide, chemical industry, organic synthesis and many other fields. In addition, the invention has the advantages of simple operation, mild reaction conditions, low cost, high yield and good application prospect.
Owner:JIYANG COLLEGE OF ZHEJIANG A & F UNIV
Preparation method of 1,5-disubsituted tetrazole compound
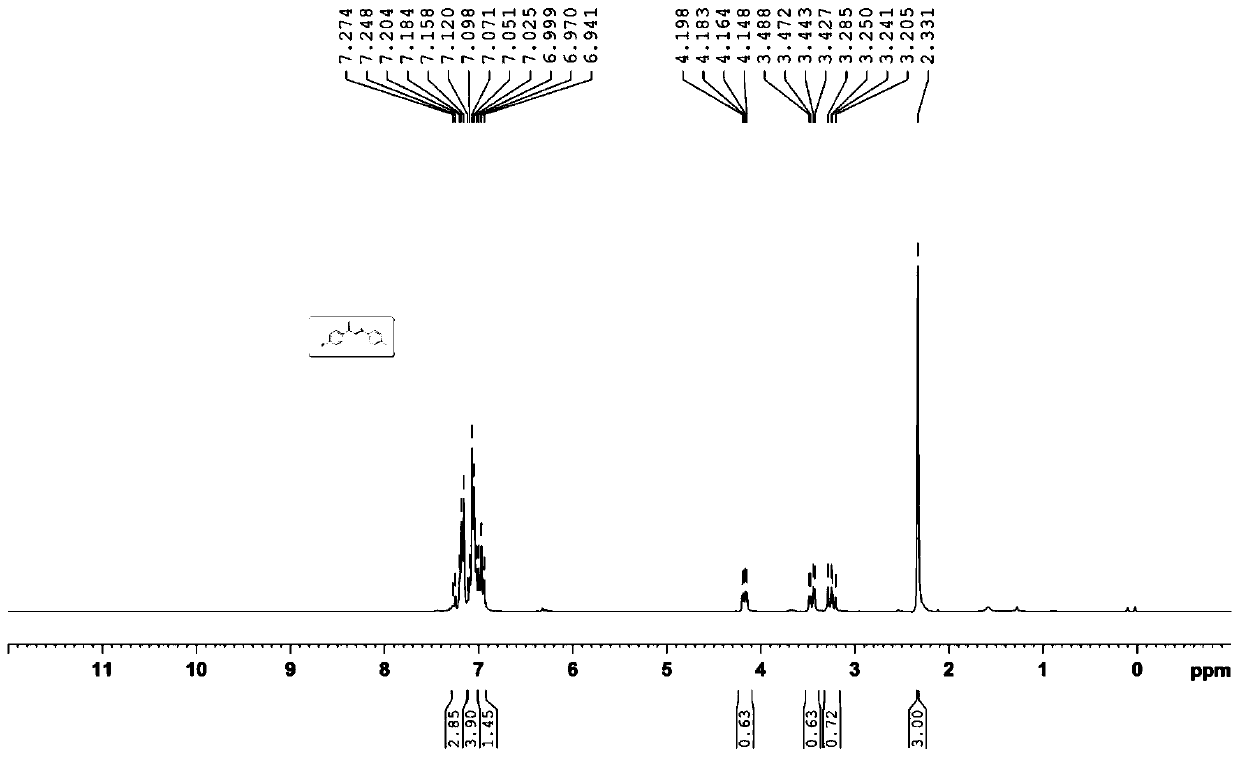
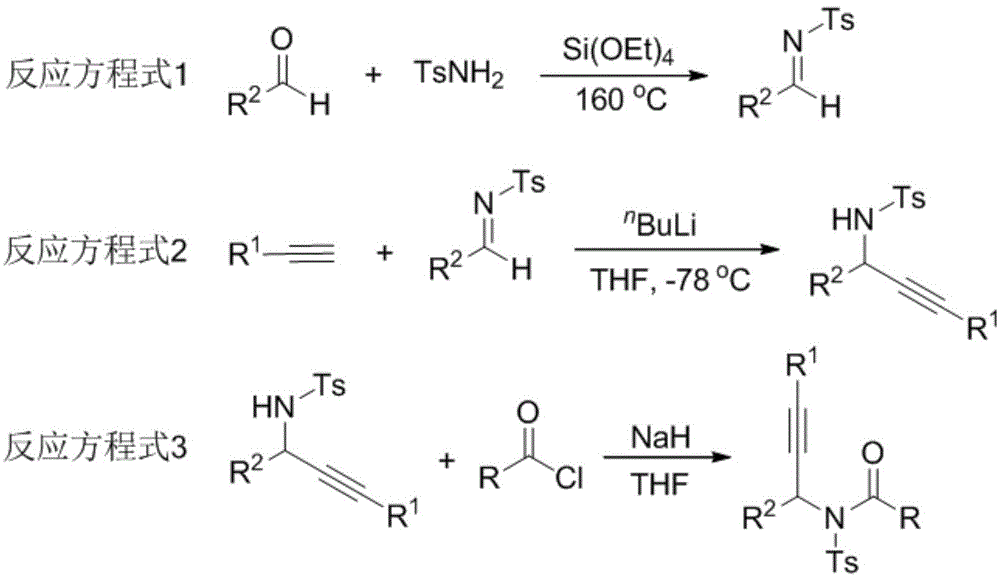
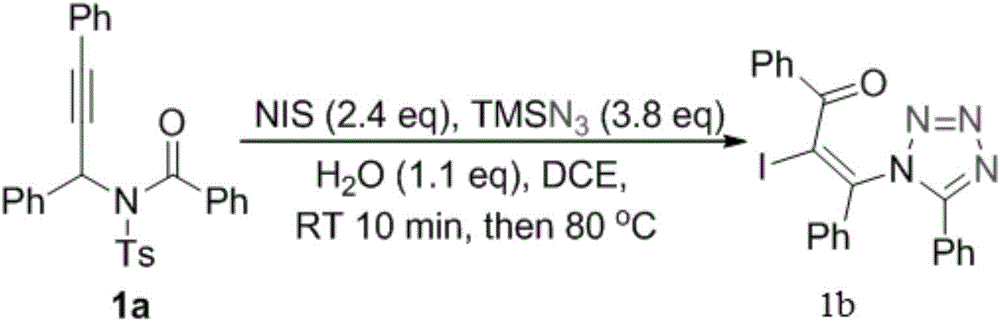
The invention relates to a preparation method of 1,5-disubsituted tetrazole compound. The method concretely comprises the following steps: propynoic acid amide which is obtained by simple preparation is used as a raw material, in a condition with N-Iodosuccinimide (NIS), azidotrimethylsilane (TMSN3) and water, an intramolecular oxygen atom transfer reaction is carried out, and the 1,5-disubsituted tetrazole compound is obtained. Propynoic acid amide is simply prepared from cheap and easily available initial raw materials, TMSN3 is used as a nitrogen source, the one-step intramolecular oxygen atom transfer reaction is carried out, and 1,5-disubsituted tetrazole is obtained. The method has the advantages of simple operation, and mild reaction conditions without metal catalysts.
Owner:中科榆林能源技术运营有限责任公司
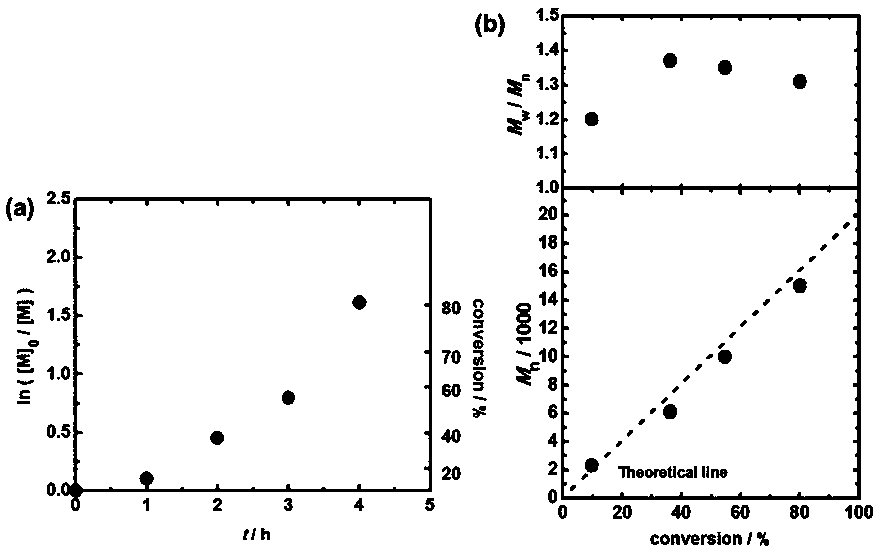
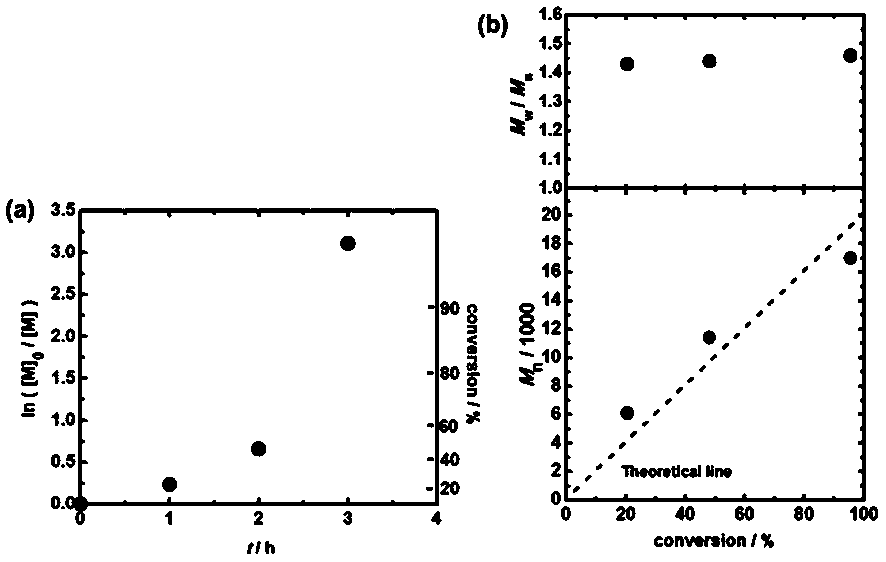
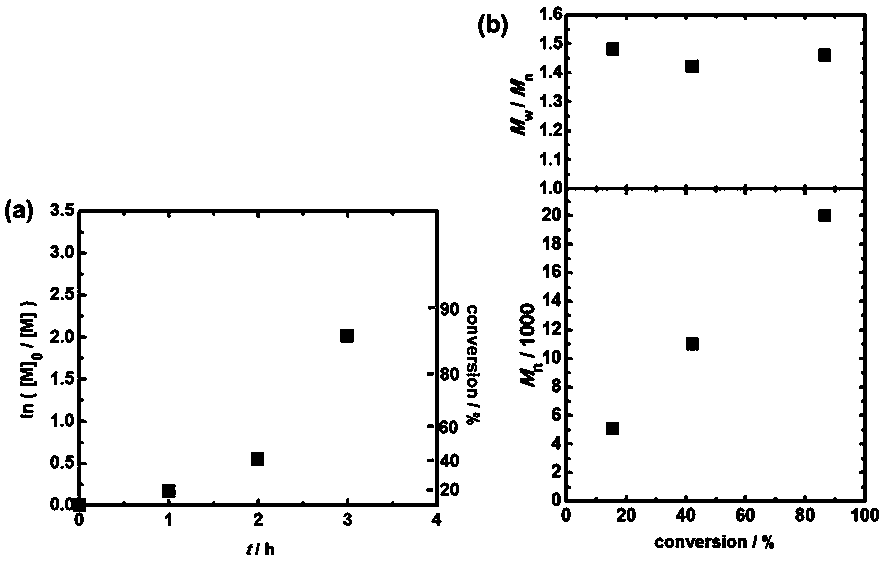
Method for realizing controllable free radical polymerization based on Finkelstein reaction
The invention discloses a method for realizing controllable free radical polymerization based on a Finkelstein reaction. The Finkelstein reaction is a reaction that a bromohydrocarbon is converted toan iodohydrocarbon with sodium iodide in polymeric monomers. The method comprises the following steps: firstly mixing an organic bromide, sodium iodide and a catalyst N-iodosuccinimide, then adding reaction monomers into the mixture, and then adding a certain amount of an azo compound and performing even mixing, then introducing nitrogen or argon to remove the air in the reaction system, and thencarrying out a polymerization reaction at a certain temperature. In the polymerization reaction process, the conversion ratio of the monomers increases with the increase of the reaction time, and a linear growth relationship exists between the molecular weight of the obtained polymer and the conversion ratio of the monomers, the dispersion coefficient of the obtained polymer is between 1.2 and 1.5, so that the polymerization reaction is a new controllable free radical polymerization reaction.
Owner:FUZHOU UNIV
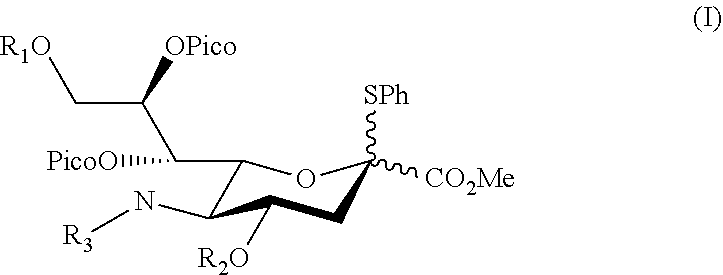
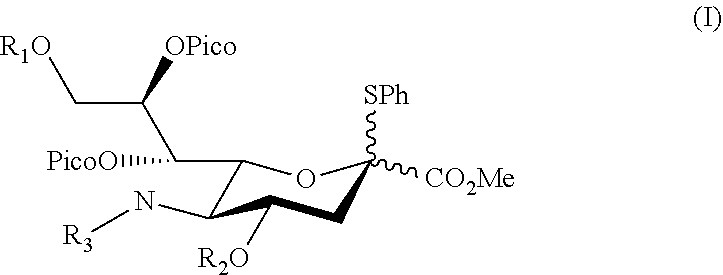
Alpha-selective sialyl donor and its uses for preparation of sialosides
ActiveUS10435425B1High stereoselectivityImprove production yieldEsterified saccharide compoundsSugar derivativesMolecular sieveGlycoside
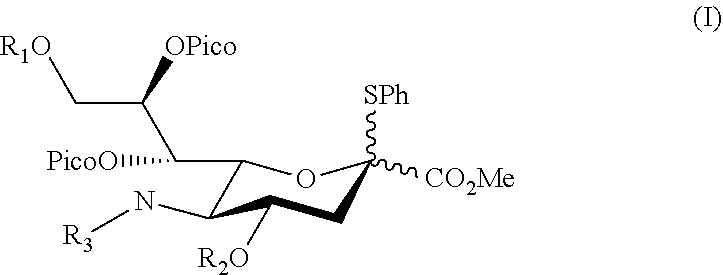
Disclosed herein a sialyl donor and its use for the synthesis of gangliosides. The sialyl donor has the structure of,wherein, R1 and R2 are independently benzoyl, toluenesulfonyl, pivaloyl or acetyl optionally substituted with a halogen; and R3 is acetyl or —(O)CCH2OH. In one preferred embodiment, in the sialyl donor of formula (I), R is acetyl. Also disclosed herein is a method of synthesizing a sialoside. The method comprises steps of: coupling the sialyl donor of formula (I) with a glycosyl acceptor having a primary hydroxyl group in the presence of N-iodosuccinimide (NIS) and trifluoromethanesulfonic acid (TfOH) under suitable conditions; and isolating the sialoside, which has an α-glycosidic linkage. According to preferred embodiments, the coupling is conducted in a solvent selected from the group consisting of, CH3CN, CH3Cl, and CH2Cl2 at a temperature between −20° C. to −60° C. Additionally or optionally, the coupling is conducted in CH2Cl2 with the presence of a powdered molecular sieve at −40° C.
Owner:CHUNG YUAN CHRISTIAN UNIVERSITY
3-Iodo-6-chloro-imidazo [1,2-a] pyridine synthetic method
InactiveCN104910155AReaction raw materials are readily availableReasonable priceOrganic chemistryOrganic synthesisChloroacetaldehyde
The present invention belongs to the field of organic synthesis, and in particular relates to a 3-iodo-6-chloro-imidazo [1,2-a] pyridine synthetic method. The synthetic method comprises the following steps: 2-amino-5-chloropyridine, A chloroacetaldehyde aqueous solution, and N-iodosuccinimide are used as raw materials for continuous reaction under the action of a base in a suitable solvent at 40-150 DEG C to obtain a 3-iodo-6-chloro-imidazo [1,2-a] pyridine crude product, wherein the ratio of the number of moles of 2-amino-5-chloropyridine to chloroacetaldehyde aqueous solution is 1: 0.7-2.8, the ratio of the number of moles of 2-amino-5-chloropyridine to N-iodosuccinimide is 1: 1.0-3.1, and the 3-iodo-6-chloro-imidazo [1,2-a] pyridine crude product is purified to obtain a 3-iodo-6-chloro-imidazo [ 1,2-a] pyridine pure product. Reaction raw materials are relatively easy to obtain, and are reasonable in prices, the reaction are mild in conditions, easy to operate, and easy to control, post-processing is simple, product quality is stable, and purity is high.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Preparation method of 2-chlorine-7-iodothieno[3,2-D] pyrimidine Preparation method of 2-chlorine-7-iodothieno[3,2-D] pyrimidine](https://images-eureka.patsnap.com/patent_img/6df4318b-4c75-41f2-bd8b-2b20e5d678af/BSA00000799403500021.PNG)

![Process for preparing 3-iodine-6-(trifluoromethyl) imidazo [1,2-a] pyridine Process for preparing 3-iodine-6-(trifluoromethyl) imidazo [1,2-a] pyridine](https://images-eureka.patsnap.com/patent_img/42cdd3f8-5bc0-4950-b842-2a99e4a444b8/BDA0001281801180000021.png)

![3-Iodo-6-chloro-imidazo [1,2-a] pyridine synthetic method 3-Iodo-6-chloro-imidazo [1,2-a] pyridine synthetic method](https://images-eureka.patsnap.com/patent_img/b9f8665d-89c3-40a8-b6c3-456760913d3e/DEST_PATH_IMAGE001.PNG)