Preparation and medical application of binary structure sinomenine derivatives
A dual structure, sinomenine technology, applied in the field of medicine, to achieve the effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of 1-iodosinomenine
[0031]
[0032] Dissolve 990 mg (3 mmoL) of sinomenine in 20 mL of dichloromethane (DCM), add 739.5 mg (3.3 mmoL) of NIS in two portions, stir at room temperature for 4 h, and detect by TLC until the reaction is almost complete. After most of the solvent was evaporated, 50 mL of ethyl acetate was added, and 2×20 mL of NH 4 Cl water was washed, and the organic layer was washed with 20 mL of saturated brine and washed with anhydrous Na 2 SO 4 After drying, the solvent was evaporated and purified by silica gel column (CHCl 3 :CH 3 OH=10:1), 835.0 mg of light yellow solid 1-iodosinomenine was obtained, the yield was 84.6%, ESI-MS: m / z 456.1 [M+H] + .
Embodiment 2
[0033] Example 2: Preparation of binary structure sinomenine derivatives
[0034]
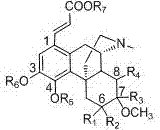
[0035] 1.41 g (3.1 mmol) of 1-iodosinomenine, triphenylphosphine (PPh 3 ) 280 mg (0.31 mmol), palladium acetate (Pd(OAc) 2 ) 35 mg (0.16 mmol) was added to 35 mL DMF-triethylamine (DMF-Et 3 N) (3:1) in the mixture, add N after evacuating for 15 min 2 , then evacuate for 15 min, inject 1.8 mL (5 eq) of ethyl acrylate and add N 2 , reacted at 80°C for 3 h. After the reaction, add 80 mL of water, extract with 100 mL×3 ethyl acetate, combine the organic layers and wash with 50 mL×3 saturated brine, combine the aqueous layers for 3 times, extract with 50 mL ethyl acetate, and extract all the organic layers Combine and dry. Most of the solvent was evaporated to 10 mL, and it was left to stand in the refrigerator overnight, and 0.5 g of a light yellow solid was precipitated. After the mother liquor was evaporated to dryness, it was purified by a silica gel column to obtain 0.4 g of a solid, an...
Embodiment 3
[0051] Example 3: Inhibition of NF-κB Activity by Binary Sinomenine Derivatives
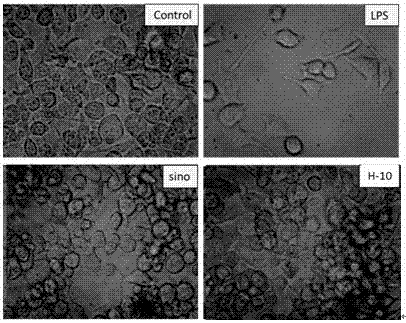
[0052] The 293 cells stably transfected with pNF-κB-luc (Shanghai Institute of Cell Biology, Chinese Academy of Sciences) were used at 1×10 5 The cells / well were inoculated into 96-well plates, and 12.5 and 50 μg / mL of drugs or DMSO were added, respectively. After adding the drugs for 15 minutes, 10 μg / mL of LPS was added to stimulate the cells for 6 hours. The luciferase activity was determined with a kit (Promega). The luminescence value was detected on the computer (Berthold Company, Sirius single-tube light detection system). Triptolide (LGT, China National Institute of Pharmaceutical and Biological Products, purity ≥ 99%) was used as a positive control. See Table 1 for specific data.
[0053] Table 1. Inhibitory effects of sinomenine and its derivatives on NF-κB signaling pathway
[0054]
[0055] ** p * p a) blank control, b) positive control
[0056] Some derivatives have si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com