Method for synthesizing (2-iodine-2-aryl) ethyl aryl ether derivatives
A technology of ethyl aryl ether and synthesis method, which is applied in thioether preparation, organic chemistry and other directions, can solve problems such as difficulty in obtaining β-iodothioether compounds, and achieves a wide range of substrate expansion and group tolerance. The effect of good sex and considerable profit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
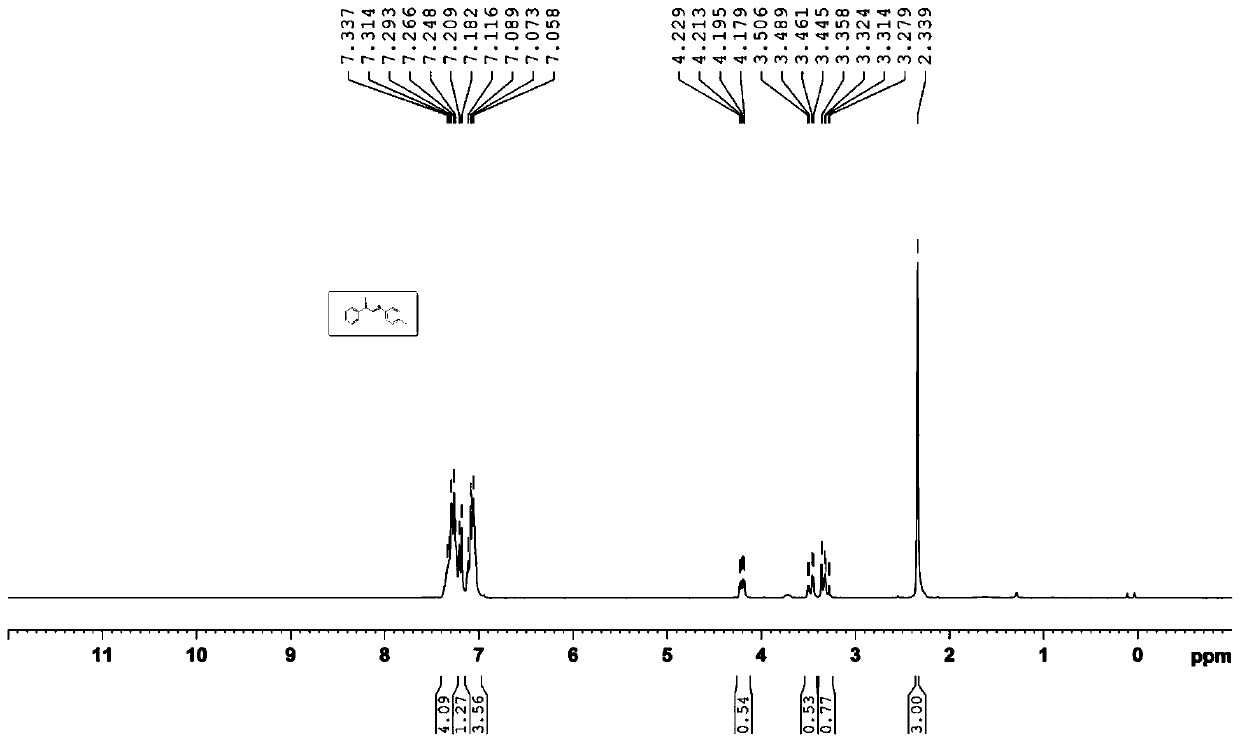
[0023] Embodiment 1: the synthesis of (2-iodo-2-phenyl) ethyl p-cresyl ether
[0024]
[0025] Add styrene (0.4mmol, 41.6mg), N-iodosuccinimide (0.1mmol, 22.5mg), p-methyldiphenyl disulfide (0.2mmol, 49.2mg), iodine Cuprous chloride (0.4eq, 7.6mg), o-phenanthroline (0.4eq, 7.9mg), 1,2-dichloroethane (2mL), magnetically stirred at 80°C for 1 hour; Extract with ethyl ester, combine the organic phases, evaporate most of the solvent under reduced pressure to obtain the crude product, use petroleum ether: ethyl acetate (20:1) as eluent to carry out column chromatography separation and purification to the crude product, and obtain the target product It is a light yellow solid with a yield of 68%.
[0026] Its NMR data are as follows:
[0027] 1 H NMR (300MHz, CDCl 3 )δ=7.34~7.25(m, 4H), 7.21~7.18(d, J=8.1Hz, 1H), 7.12~7.06(m, 4H), 4.23~4.18(dd, J=4.8Hz, J=10.2Hz ,1H), 3.51~3.45(dd, J=5.1Hz, J=13.5Hz, 1H), 3.36~3.28(dd, J=10.2Hz, J=13.2Hz, 1H), 2.34(s,3H).
Embodiment 2
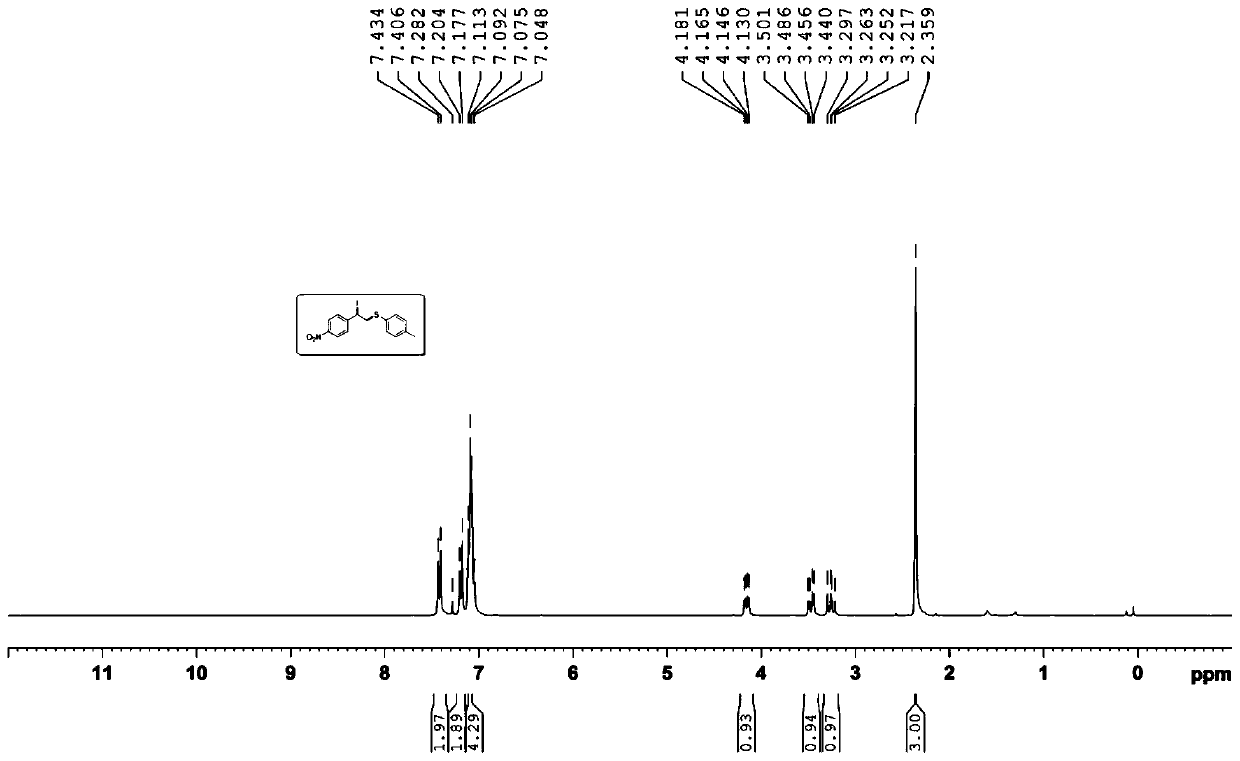
[0028] Embodiment 2: the synthesis of (2-iodo-2-(4'-nitrophenyl)) ethyl p-cresyl ether
[0029]
[0030] Add 4-nitrostyrene (0.4mmol, 59.6mg), N-iodosuccinimide (0.1mmol, 22.5mg), p-methyldiphenyl disulfide (0.2mmol, 49.2 mg), cuprous iodide (0.4eq, 7.6mg), o-phenanthroline (0.4eq, 7.9mg), 1,2-dichloroethane (2mL), magnetically stirred at 80°C for 1 hour; Finally, extract with ethyl acetate, combine the organic phases, evaporate most of the solvent under reduced pressure to obtain the crude product, use sherwood oil: ethyl acetate (10:1) as the eluent to carry out column chromatography separation and purification for the crude product, The target product was obtained as a brown-yellow solid with a yield of 70%.
[0031] Its NMR data are as follows:
[0032] 1 H NMR (300MHz, CDCl 3 )δ=7.42(d, J=8.1Hz, 2H), 7.19(d, J=8.1Hz, 2H), 7.11~7.05(m, 4H), 4.18~4.13(dd, J=4.8Hz, J=10.5 Hz, 1H), 3.50~3.44(dd, J=4.5Hz, J=13.5Hz, 1H), 3.30~3.22(dd, J=10.2Hz, J=13.5Hz, 1H), 2.36(s, 3H...
Embodiment 3
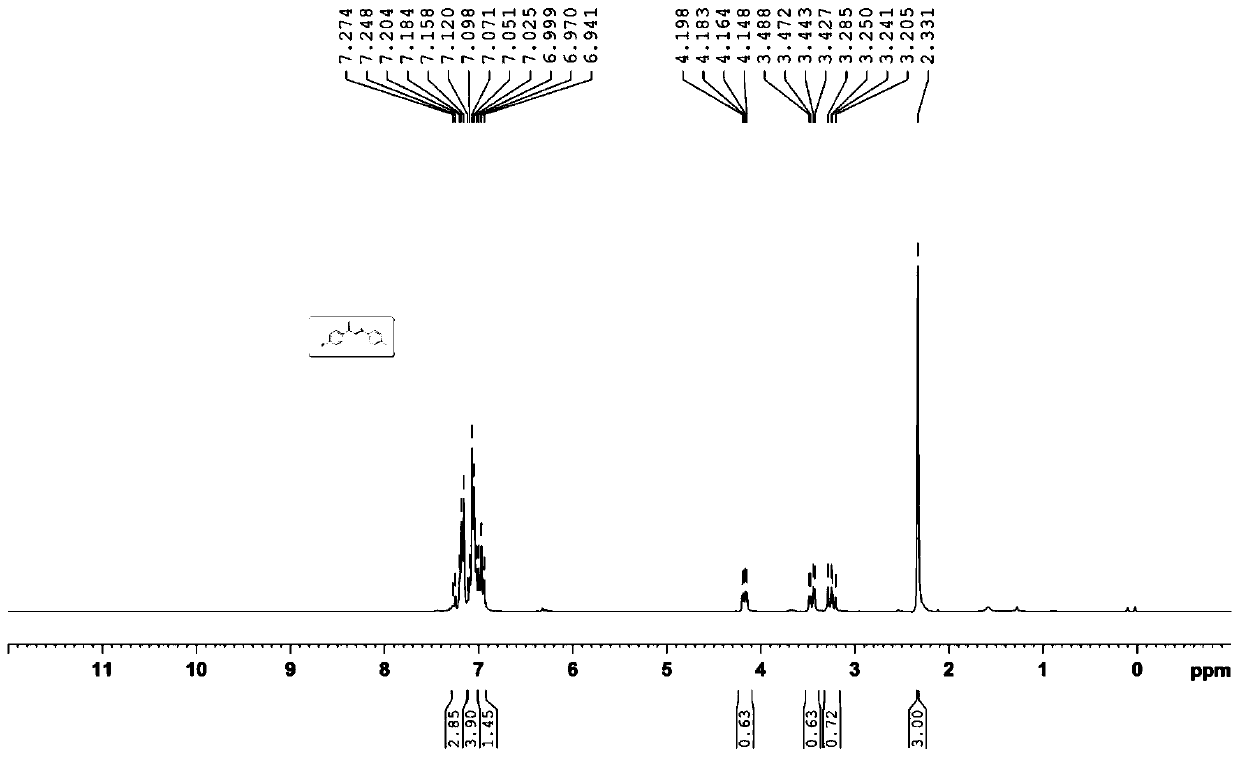
[0033] Embodiment 3: the synthesis of (2-iodo-2-(4'-fluorophenyl)) ethyl p-cresyl ether
[0034]
[0035] Add 4-fluorostyrene (0.4mmol, 48.8mg), N-iodosuccinimide (0.1mmol, 22.5mg), p-methyldiphenyl disulfide (0.2mmol, 49.2mg) into a 25mL reaction tube ), cuprous iodide (0.4eq, 7.6mg), o-phenanthroline (0.4eq, 7.9mg), 1,2-dichloroethane (2mL), magnetically stirred at 80°C for 1 hour; , extract with ethyl acetate, combine the organic phases, evaporate most of the solvent under reduced pressure to obtain the crude product, use petroleum ether: ethyl acetate (10:1) as eluent to carry out column chromatography separation and purification to the crude product, obtain The target product is a light yellow solid with a yield of 70%.
[0036] Its NMR data are as follows:
[0037] 1 H NMR (300MHz, CDCl 3 )δ=7.27~7.18(m,3H),7.16~7.07(m,4H),7.05~6.94(m,1H),4.20~4.15(dd,J=4.5Hz,J=10.2Hz,1H),3.49 ~3.43 (dd, J=4.8Hz, J=13.5Hz, 1H), 3.29~3.21 (dd, J=10.2Hz, J=13.5Hz, 1H), 2.33 (s, 3H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com