Preparation method of 1,5-disubsituted tetrazole compound
An azole compound and a disubstituted technology are applied in the field of preparation of 1,5-disubstituted tetrazole compounds and achieve the effects of mild conditions and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
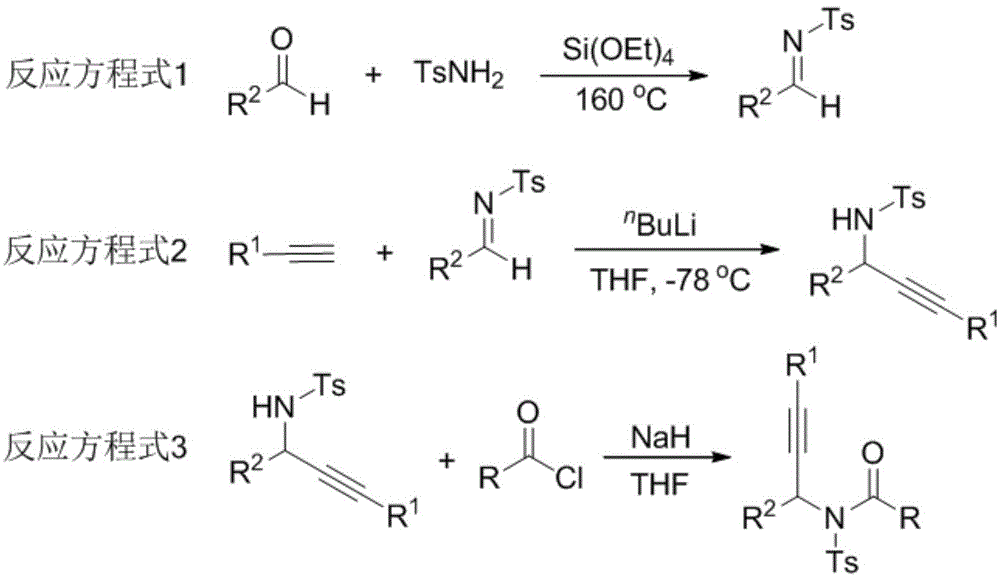
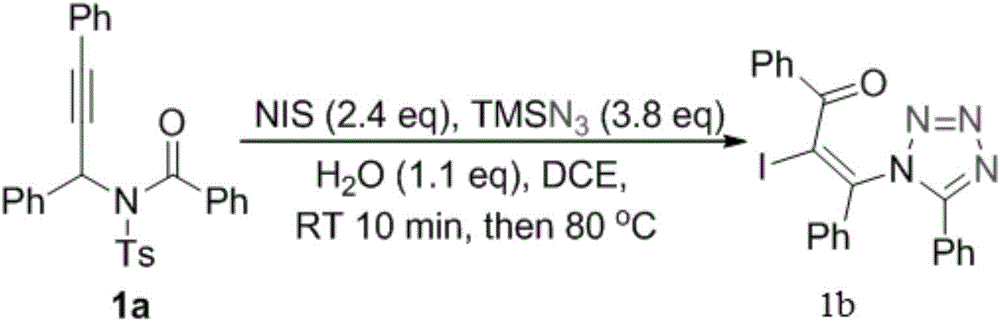
[0023] Synthesis steps of propynamide 1a: react in a 50mL three-necked reaction flask, vacuumize the reaction tube and replace it with argon three times, then add 15mmol of benzaldehyde, 10mmol of p-toluenesulfonamide and 20mmol of triethylorthosilicate , Reacted at 160°C for 10h, then recrystallized from ethyl acetate and petroleum ether to obtain a white solid aldimine. The reaction was carried out in a 50mL three-necked reaction flask. After the reaction tube was evacuated and replaced with argon three times, 6mmol of phenylacetylene was added and dissolved in 20mL of THF. At -78°C, 7mmol of butyllithium was slowly added dropwise to keep Stir at this temperature for 40min, then weigh 5mmol of imine and dissolve it in 20mL of THF, add dropwise to the reaction solution, rise to room temperature, react for 3h, extract with ethyl acetate, recrystallize with ethanol and petroleum ether to obtain light yellow solid propargyl base amine. The reaction was carried out in a 50mL thr...
Embodiment 2
[0028] Synthesis steps of propynamide 2a: react in a 50mL three-necked reaction flask, vacuumize the reaction tube and replace it with argon three times, then add 15mmol of o-chlorobenzaldehyde, 10mmol of p-toluenesulfonamide and 20mmol of orthosilicate Ethyl ester was reacted at 160°C for 10 h, and then recrystallized from ethyl acetate and petroleum ether to obtain aldimine as a white solid. The reaction was carried out in a 50mL three-necked reaction flask. After the reaction tube was evacuated and replaced with argon three times, 6mmol of phenylacetylene was added and dissolved in 20mL of THF. At -78°C, 7mmol of butyllithium was slowly added dropwise to keep Stir at this temperature for 40min, then weigh 5mmol of imine and dissolve it in 20mL of THF, add it dropwise to the reaction solution, rise to room temperature, react for 3h, extract with ethyl acetate, recrystallize with ethanol and petroleum ether to obtain light yellow solid propargyl base amine. The reaction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com