Oxathiadiazole-2-oxide compound and preparation method thereof
A compound, potassium hydroxide technology, applied in the direction of organic chemistry, etc., can solve the problems such as no synthesis report
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
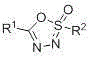
[0012] Its reaction process is attached as follows figure 2 , where R 1 , R 2 For a hydrogen atom, an alkyl group, an aryl group, etc.
[0013] Specific preparation method example 1: 0.5 mmol 4-methyl-N'-(4-nitrobenzylidene) benzenesulfonyl hydrazide, 1 mmol N-chlorosuccinimide (additive), 0.5 mmol Molar potassium hydroxide (base) and 1 ml of nitromethane were mixed together and the reaction was stirred well at 0°C for 5 hours. After the reaction was completed, it was extracted, washed with organic solvent, concentrated, and purified by column chromatography to obtain 5-p-nitrophenyl-2-p-methylphenyl-1,2,3,4-oxathiadiazole-2-oxide The yield was 89%. Melting point is 100~101°C; H NMR spectrum 1 HNMR (400MHz, CDCl 3 )δ8.27(d,2H, J =8.7Hz)7.75(d,2H, J =8.3Hz)7.66(d,2H, J =8.7Hz)7.42(d,2H, J =8.2Hz)2.51(s,3H); C NMR 13 CNMR (100MHz, CDCl 3 )δ152.2, 149.6, 146.5, 135.6, 133.5, 130.0, 129.7, 128.6, 123.5, 21.8; infrared spectrum FTIR (film): 2925, 2854, 1595, 1525, 1377...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com