4-cyano-5-(3-indolyl)oxazole compounds and preparation method thereof
A technology of indolyl and oxazoles, which is applied in the field of 4-cyano-5-oxazoles and their preparation, can solve the problems of unfriendly environment, high cost, high reaction temperature, etc., and achieve atom economy and environmental friendliness , simple steps, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of 5-(1H-indol-3-yl)-2-phenyloxazole-4-carbonitrile (1a)
[0033]
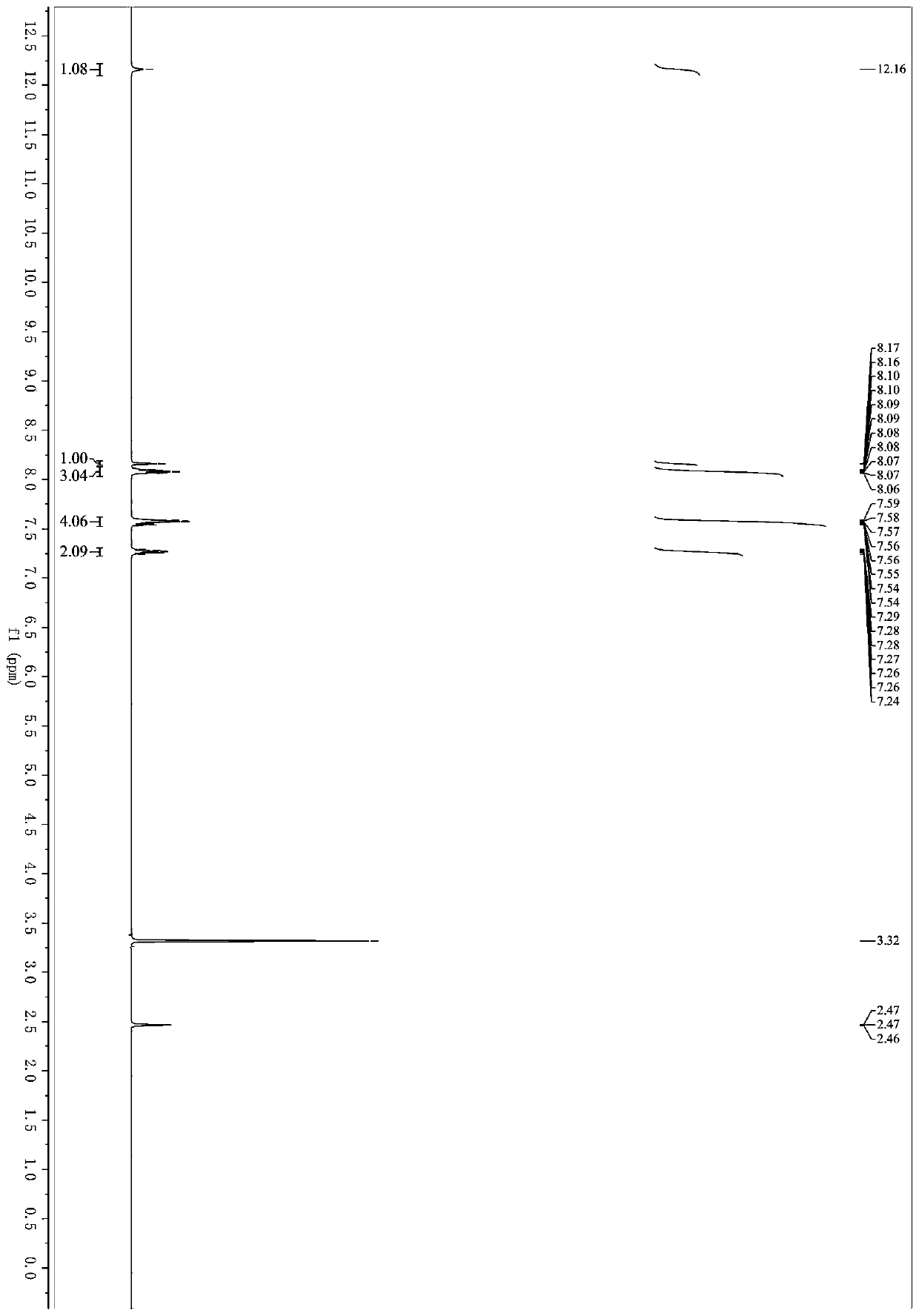
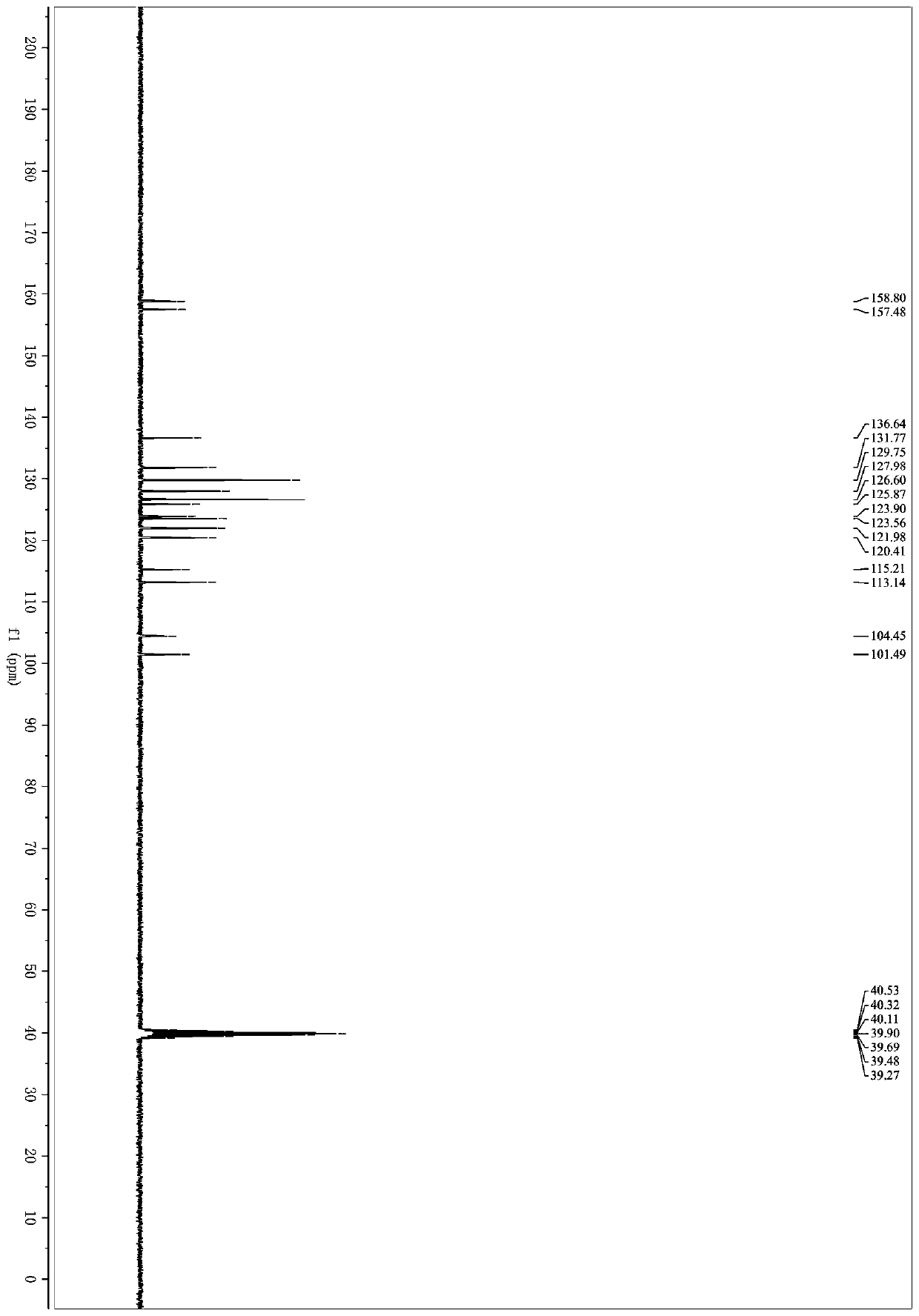
[0034] 3-(1H-indol-3-yl)-3-oxopropanecyanide (92mg, 0.5mmol), benzylamine (107mg, 1mmol), N,N-dimethylformamide (3 mL), iodine (32mg, 0.125mmol), tert-butanol peroxide (0.28mL, 70% aqueous solution, 2.0mmol) were successively added in a 25mL Schlenk bottle, placed in an oil bath for reaction, the reaction temperature was controlled at 60°C, and the reaction was carried out for 7 hours. After the reaction was over, the organic solvent was removed under reduced pressure; eluting with petroleum ether / ethyl acetate and separated on a silica gel column to obtain 5-(1H-indol-3-yl)-2-phenyloxazole-4-carbonitrile ( 1a) 110 mg, yield 77%. 1 H NMR (400MHz, DMSO-d 6 )δ12.16(s,1H),8.16(d,J=1.8Hz,1H),8.08(ddd,J=9.1,4.1,1.9Hz,3H),7.61–7.53 (m,4H),7.31–7.22 (m,2H). 13 C NMR (101MHz, DMSO-d 6 )δ158.80,157.48,136.64,131.77,129.75,127.98,126.60,125.87,123.90,123.56,121.98,120.41,115.21,113.14...
Embodiment 2
[0035] Example 2: Synthesis of 5-(1H-indol-3-yl)-2-(4-toluene)oxazole-4-carbonitrile (1b)
[0036]
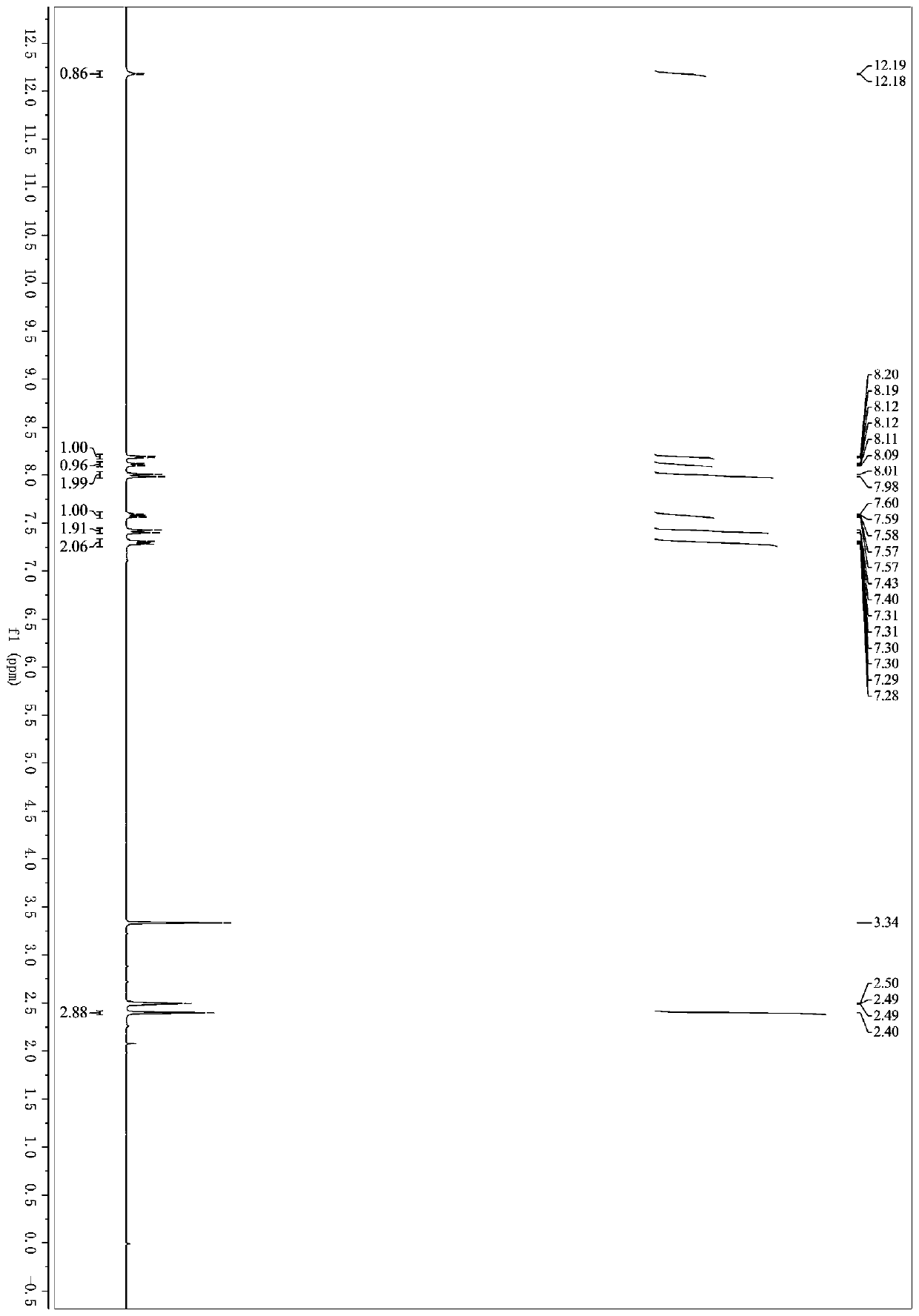
[0037] 3-(1H-indol-3-yl)-3-oxopropylcyanide (92mg, 0.5mmol), 4-methylbenzylamine (121mg, 1mmol), N,N-dimethylformamide (3mL ), iodine (32mg, 0.125mmol), and tert-butanol peroxide (0.28mL, 70% aqueous solution, 2.0mmol) were successively added into a 25mL Schlenk bottle, placed in an oil bath for reaction, and the reaction temperature was controlled at 60°C. After 7 hours, after the reaction was over, the organic solvent was removed under reduced pressure; eluted with petroleum ether / ethyl acetate, separated on a silica gel column to obtain 5-(1H-indol-3-yl)-2-(4-toluene)oxazole -4-carbonitrile (1b) 116 mg, yield 78%. 1 H NMR (300MHz, DMSO-d 6)δ12.23–12.11(m,1H),8.19(d,J=2.9Hz,1H),8.14–8.06 (m,1H),7.99(d,J=8.0Hz,2H),7.64–7.51(m ,1H),7.42(d,J=8.0Hz,2H),7.36–7.22(m,2H),2.40(s,3H). 13 C NMR (75MHz, DMSO-d 6 )δ159.16,157.27,141.99,136.70,130.40,128.00,126.68,123.96,123.61,12...
Embodiment 3
[0038] Example 3: Synthesis of 5-(1H-indol-3-yl)-2-(2-furan)oxazole-4-carbonitrile (1c)
[0039]
[0040] 3-(1H-indol-3-yl)-3-oxopropanecyanide (92mg, 0.5mmol), 2-furylmethylamine (97mg, 1mmol), N,N-dimethylformamide (3mL) , iodine (32mg, 0.125mmol), tert-butanol peroxide (0.28mL, 70% aqueous solution, 2.0mmol) were successively added in a 25mL Schlenk bottle, and placed in an oil bath for reaction. The reaction temperature was controlled at 60°C. Reaction 7 Hours, after the reaction was over, the organic solvent was removed under reduced pressure; eluted with petroleum ether / ethyl acetate, separated on a silica gel column to obtain 5-(1H-indol-3-yl)-2-(2-furan)oxazole- 4-carbonitrile (1c) 82 mg, yield 60%. 1 H NMR (300MHz, DMSO-d 6 )δ12.21(s,1H),8.18(s,1H),8.13–7.92(m,2H),7.69–7.49 (m,1H),7.43(d,J=3.5Hz,1H),7.37–7.20 (m,2H),6.81(dd,J=3.6,1.8Hz,1H). 13 C NMR (75MHz, DMSO-d 6 )δ156.92,151.66,146.95,141.15,136.69,128.15,123.93,123.67,122.04,120.45,115.03,114.11, 113.23,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com