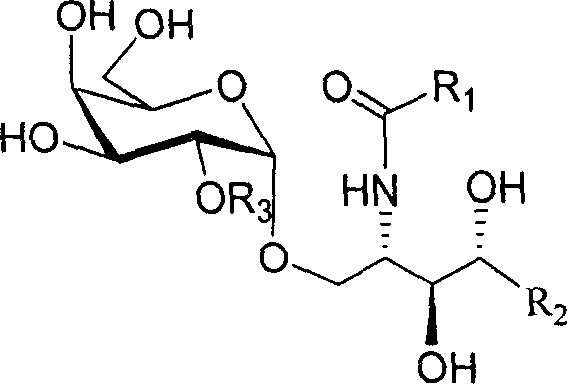
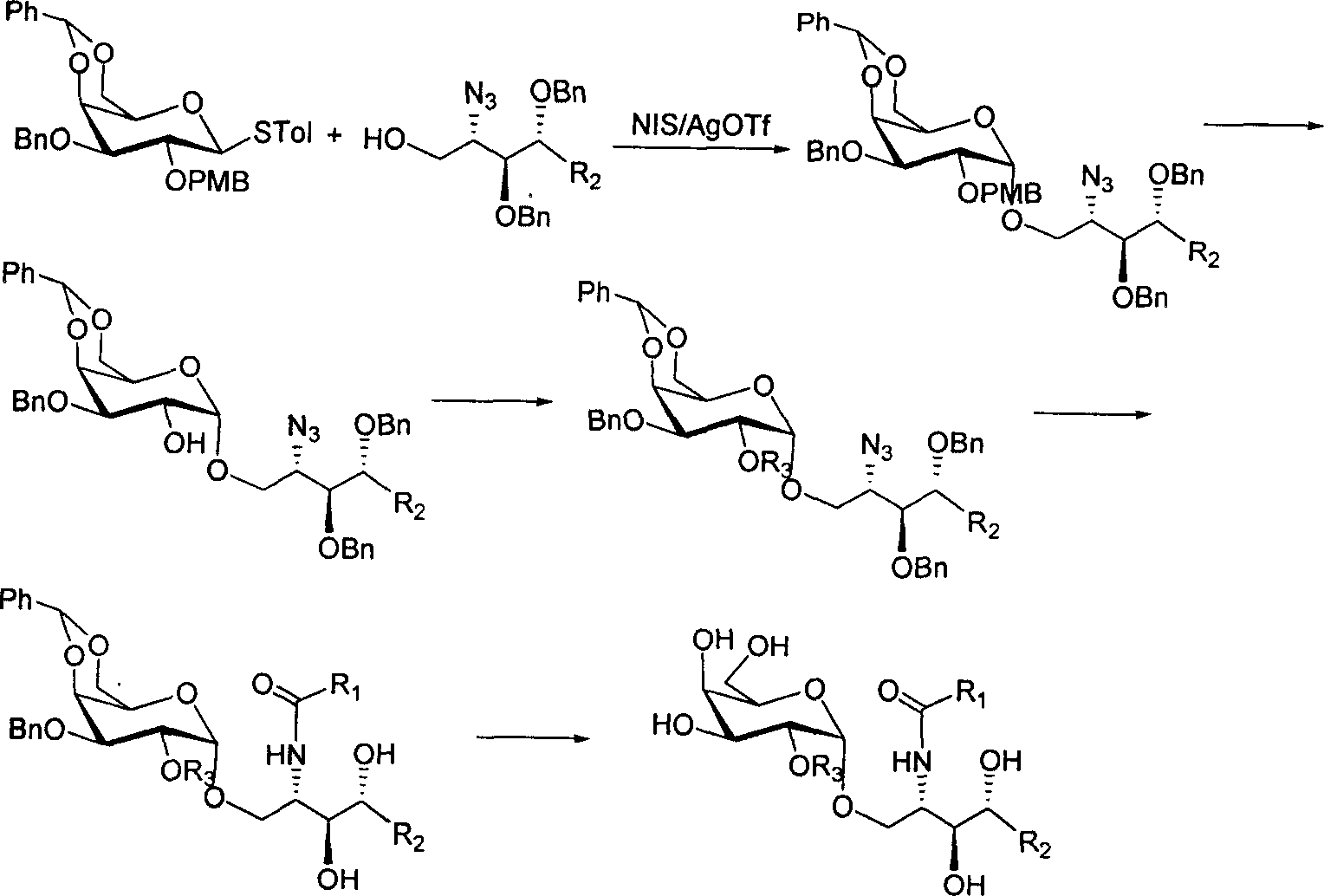
2'-OH derivative of alpha-galactose glycosphingolipids and preparing method thereof
A technology for glycosphingolipids and derivatives, which is applied in the field of 2'-OH derivatives of α-galactosphingolipids and their preparation, can solve the problems of different configuration ratios of glycosylation products, difficulties in purification, separation, identification, and limitations. Glycosphingolipid research progress and other issues, to achieve the effect of simple method, short route and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0009] First, glycosidation reaction: 2.4mmol glycosyl donor 3-O-benzyl-4,6-O-benzylidene-2-O-p-methoxybenzyl-p-methylthioglucoside, 2.0mmol containing Add phytosphingosine acceptors with 5-18 carbon atoms and newly activated 4 Å molecular sieves into a mixed solvent of 63mL dichloromethane, diethyl ether, and tetrahydrofuran (the volume ratio of the three is 1:5:1), and cool to -20°C. Under the protection of argon, 3.2 mmol of N-iodosuccinimide (NIS) and 0.2 mmol of catalyst silver trifluoromethanesulfonate (AgOTf) were sequentially added. The reaction solution was stirred for 30 minutes and then placed at 0° C. for glycosylation reaction for 12 hours. Filter off the molecular sieves, the filtrate is concentrated, and the residue obtained after concentration is dissolved in 50mL of dichloromethane, followed by 10% (weight percent concentration, the same below) Na 2 S 2 o 3 aqueous solution, washed with saturated brine, and the dichloromethane organic layer was dried over ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com