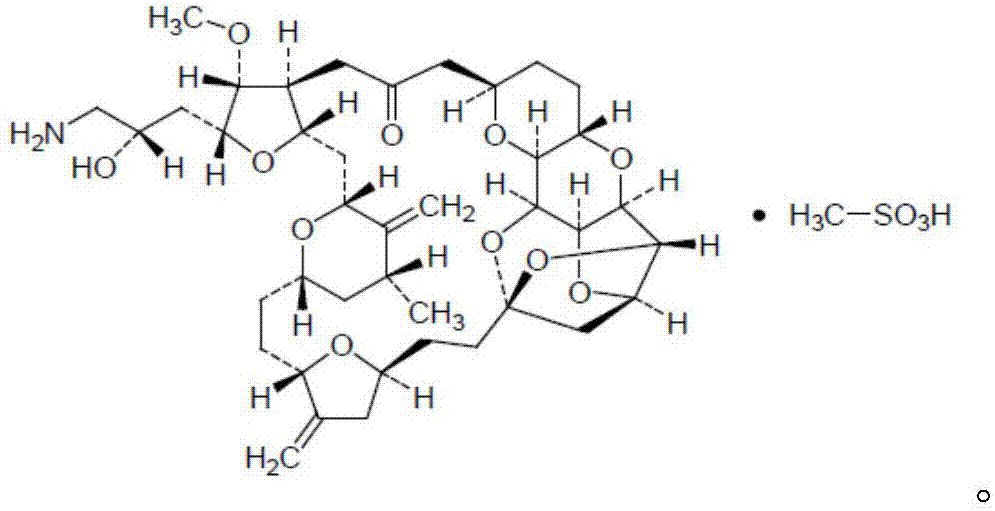
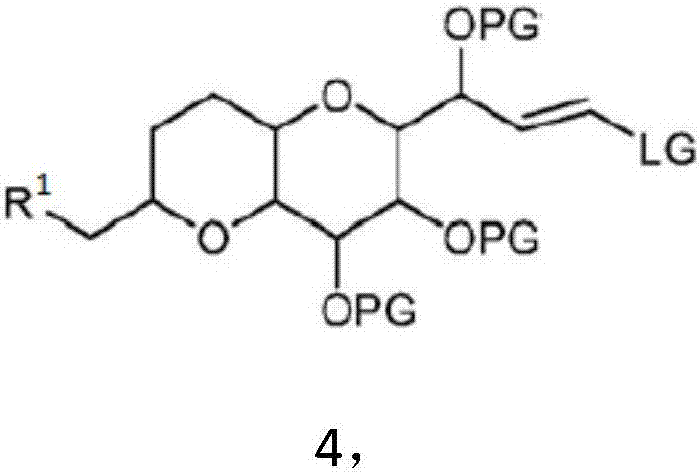
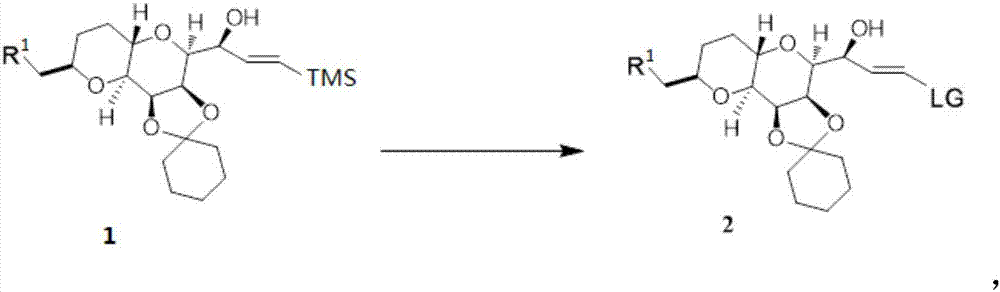
Synthesis method of eribulin intermediate
A synthetic method and a suitable technology are applied in the field of synthesis of eribulin intermediates, which can solve the problems of difficult post-processing and purification, unfavorable industrial production, troublesome quality control, etc., and achieve simple and easy post-processing operations, improved yield and purity. , The effect of simple operation and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Step 1) Nitrogen protection in the reactor, add 14ml of toluene solution at room temperature, 4.54g (0.01mol) of the compound shown in formula 1, 22ml of acetonitrile solution, and then add a catalytic amount of 100mg (0.0007mol) tert-butyldimethyl Chlorosilane TBSCl, stirred for 30 minutes, then added N-iodosuccinimide NIS 6.92g (0.03mol) at 25°C, continued to stir for 2 hours, TLC detected that the reaction was complete, and added 8.4g of sodium bicarbonate to neutralize the reaction, Extracted with 100ml of ethyl acetate, washed with 100ml of brine, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 5.07g of the compound represented by formula 6 as an oily product with a yield of 97%, which was directly put into the next reaction.
[0050] Step 2) Nitrogen protection in the reactor, add 25ml of acetic acid and 25ml of water at room temperature, 5.23g (0.01mol) of the compound shown in formula 6, then heat to 95 ° C, stir for 4 hours, TLC detectio...
Embodiment 2
[0053] Step 1) Nitrogen protection in the reactor, add 14ml of toluene solution at room temperature, 4.54g (0.01mol) of the compound shown in formula 1, 22ml of acetonitrile solution, and then add a catalytic amount of 14.3mg (0.0001mol) tert-butyl dimethyl Chlorosilane TBSCl, stirred for 30 minutes, then added 4.61g (0.02mol) of N-iodosuccinimide NIS at 20°C, continued to stir for 4 hours, TLC detected that the reaction was complete, and added 8.4g of sodium bicarbonate to neutralize the reaction , extracted with 100ml ethyl acetate, washed with 100ml brine, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 5.06g of the compound shown in formula 6 as an oily product with a yield of 96.8%, which was directly put into the next reaction.
[0054] Step 2) Nitrogen protection in the reactor, add 25ml of acetic acid and 25ml of water at room temperature, 5.23g (0.01mol) of the compound shown in formula 6, then heat to 90 ° C, stir for 2 hours, TLC detection re...
Embodiment 3
[0057] Step 1) Nitrogen protection in the reactor, add 14ml of toluene solution at room temperature, 4.54g (0.01mol) of the compound shown in formula 1, 22ml of acetonitrile solution, and then add a catalytic amount of 14.3mg (0.0001mol) tert-butyl dimethyl Chlorosilane TBSCl, stirred for 30 minutes, then added N-iodosuccinimide NIS 4.61g (0.02mol) at 30°C, continued to stir for 3 hours, TLC detected that the reaction was complete, and added 8.4g of sodium bicarbonate to neutralize the reaction , extracted with 100ml ethyl acetate, washed with 100ml brine, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 5.1g of the compound shown in formula 6 as an oily product with a yield of 97.5%, which was directly put into the next reaction.
[0058] Step 2) Nitrogen protection in the reactor, add 30ml of acetic acid and 30ml of water at room temperature, 5.23g (0.01mol) of the compound shown in formula 6, then heat to 100 ° C, stir for 6 hours, TLC detects that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com