Extended type fluorescent nucleoside analog and preparing method thereof
A technology of nucleoside analogs and fluorescence, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of cumbersome post-processing, harsh conditions, and low product yields, and achieve excellent fluorescence properties, Apply a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
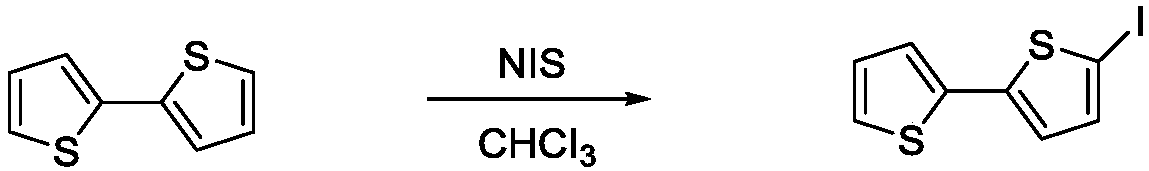
[0024] Example 1: Synthesis of 5-iodo-2,2'-dithiophene
[0025] Add 0.499g (3mmol) of 2,2'-dithiophene, 0.810g (3.6mmol) of N-iodosuccinimide and 30ml of chloroform into the round-bottomed flask respectively, then stir and raise the temperature to 20~50℃ to react for 7 hours . After cooling to room temperature, adding 5 ml of deionized water to precipitate impurities, and then extracting with ethyl acetate (3×5 ml), after rotary evaporation, 0.75 g of white solid was obtained, and the yield was 86%.
example 2
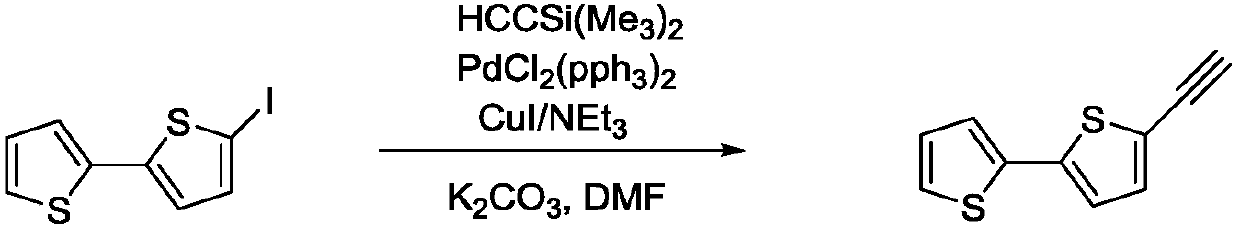
[0026] Example 2: Synthesis of 2,2'-dithiophene acetylide
[0027] Under the protection of nitrogen, add the crude product 5-iodo-2,2'-dithiophene, 25ml N,N-dimethylformamide, 0.46ml (3.6mmol) trimethylsilicon into the reaction tube. Acetylene, 0.08g (0.12mmol) of bistriphenylphosphorus palladium dichloride, 0.11g (0.57mmol) of cuprous iodide, and 1,7ml (12mmol) of triethylamine, the temperature is raised to 50-70°C and reacted for 4 hours. After the temperature dropped to room temperature, 1 g (4.5 mmol) of potassium carbonate was added, and the mixture was stirred at room temperature for 3 hours, and the solution was light brown. After filtration and rotary evaporation, 10 ml of deionized water was added, and the mixture was extracted with ethyl acetate (3×5 ml). After the combined organic phases were rotary evaporated, 0.39 g of light brown solid was obtained, and the yield was 83%.
example 3
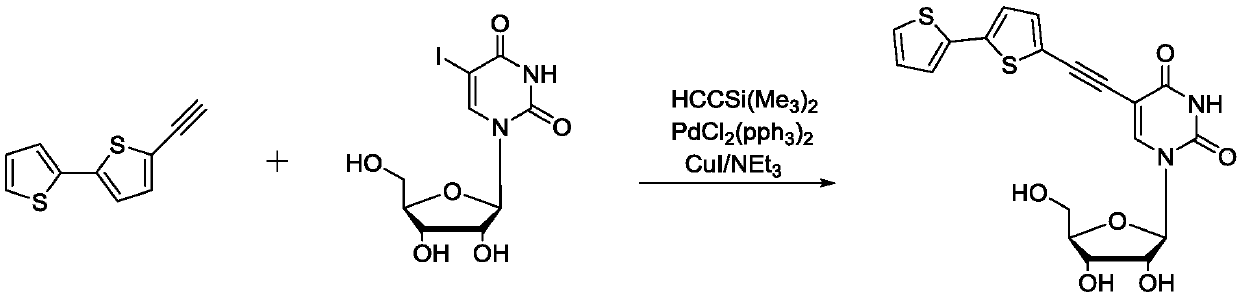
[0028] Example 3: Synthesis of fluorescent nucleoside analogues
[0029] Under the protection of nitrogen, add the 2,2'-dithiophene acetylide obtained in the previous step, 0.37g (1mmol) 5-iodouridine, and 0.06g (0.05mmol) bistriphenylphosphonium dichloride into the reaction tube. Palladium, 0.01g (0.07mmol) of cuprous iodide, 0.7ml (5.3mmol) of triethylamine and 20ml of N,N-dimethylformamide, react at 65-70°C for 4 hours. The solid was removed by filtration, the filtrate was concentrated and diluted with 10 ml of dichloromethane, washed with saturated brine three times, and the organic phase was dried over anhydrous sodium sulfate overnight. After filtration and rotary evaporation, the nucleoside derivative 0.60 g (light yellow solid) was obtained by passing through the column with a methanol / dichloromethane mixed solvent, and the yield was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com