Synthesis method of JAK kinase inhibitor intermediate
A technology of intermediates and compounds, applied in the field of medicinal chemistry, can solve the problems that N-iodosuccinimide is expensive, not easy to separate and purify, and not suitable for industrial production, and achieve simplified post-processing methods, stable yields, The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
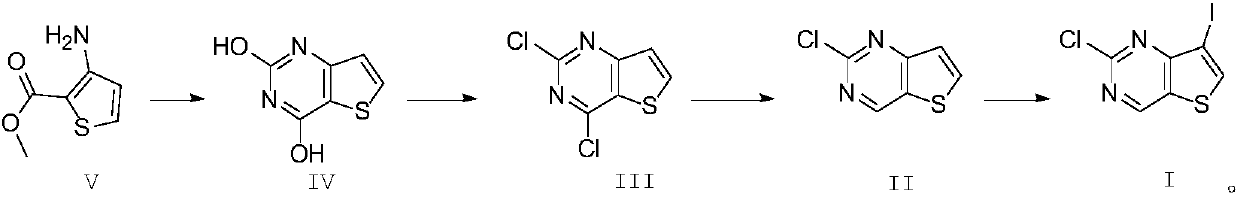
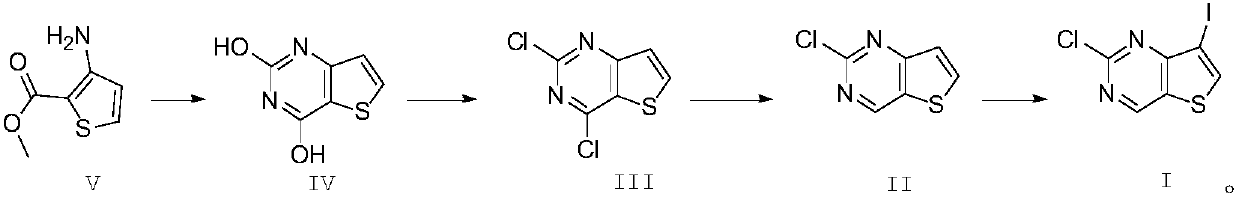
[0032] 1) Synthesis of formula IV compound:
[0033] Add 500g of the compound of formula V and 800g of urea into a dry three-necked flask, stir, heat to 190°C, and stir for 4 hours. After the reaction is completed, add it to 1600ml of ice-saturated aqueous sodium hydroxide solution while hot, stir overnight, and filter , the filtrate was adjusted to pH 2 with hydrochloric acid, filtered, the filter cake was washed once with water, and dried to obtain 481g of the compound of formula IV, HPLC: 97%.
[0034] 2) Synthesis of formula III compound:
[0035] Add 400g of the compound of formula IV to 1500ml of thionyl chloride, add 10ml of DMF, and heat to reflux for 10h. The pH of the solution was adjusted to neutral, extracted with dichloromethane, washed with saturated aqueous sodium bicarbonate solution, backwashed with saturated brine, dried, and distilled under reduced pressure to obtain 450 g of intermediate III, HPLC: 95%.
[0036] 3) synthesis of formula II compound:
[00...
Embodiment 2
[0041] Add 80g of N-chlorosuccinimide to 600ml of dimethylacetamide, control the temperature at 25°C, then add 90g of KI, stir for 2.5 hours after the addition, then add 50g of intermediate II in batches, add After completion, the temperature was raised to 100° C., the reaction was completed and cooled to room temperature, water was added, a large amount of solids were precipitated, filtered by suction, and dried to obtain 82 g of product I with a yield of 95%, HPLC: 99%.
Embodiment 3
[0043] Add 150g of N-chlorosuccinimide to 800ml DMSO, control the temperature at 30°C, then add 180gNaI, stir for 4 hours after the addition, then add 100g of intermediate II in batches, after the addition is complete, heat up to 90 ℃. After the reaction was completed, cooled to room temperature, added water, a large amount of solids were precipitated, filtered with suction, and dried to obtain 161g of product I with a yield of 92%, HPLC: 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com