Bispecific antibody biological activity and titer detection method and application thereof
A bispecific antibody and biological activity technology, which is applied in the field of bispecific antibody biological activity and titer detection, can solve the problems of bispecific antibody inapplicability and inability to screen out bispecific antibody, so as to save experiments Effects of reagent cost, manpower reduction, and low reaction volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1, for detecting the integrity and biological activity of the light chain:
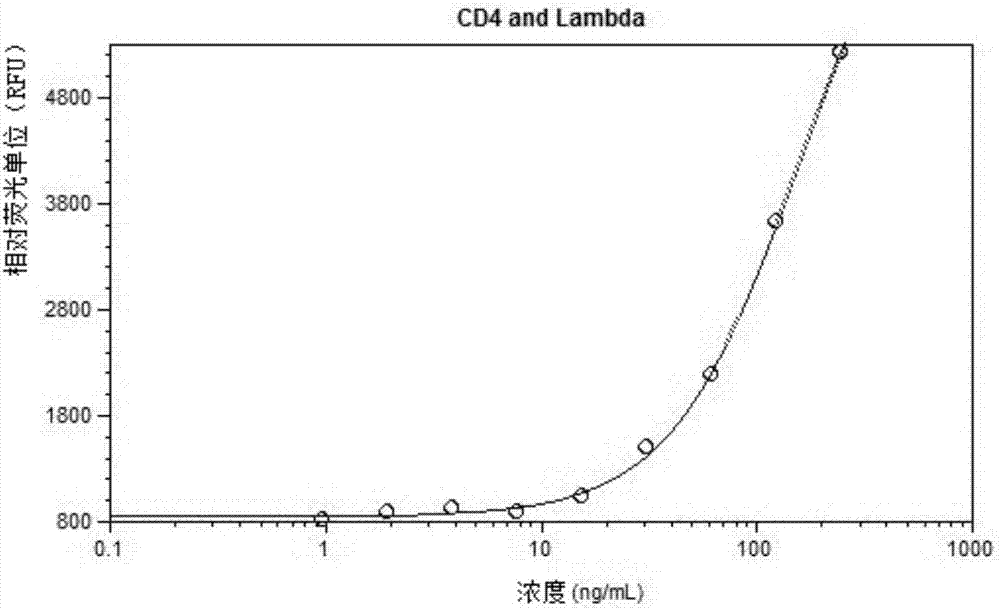
[0089] 1) Add cell supernatant, Anti-Lambda-Eu in 384 microwell plate 3+ cryptate (anti-Lambda light chain antibody coupled to europium crypt), Anti-his-XL665 (anti-His antibody linked to XL665), recombinant human CD4 protein with his tag (ie, antigen A), and mix well. Incubate at room temperature for 2 hours or more or overnight with anti-CD4 and anti-ENV bispecific antibodies as standard (final concentration: 0-250 ng / mL).
[0090] 2) Set the fluorescence wavelength: Excitation 313nm, Emission 665nm / 620nm, cutoff 630nm / 590nm, and read the fluorescence value.
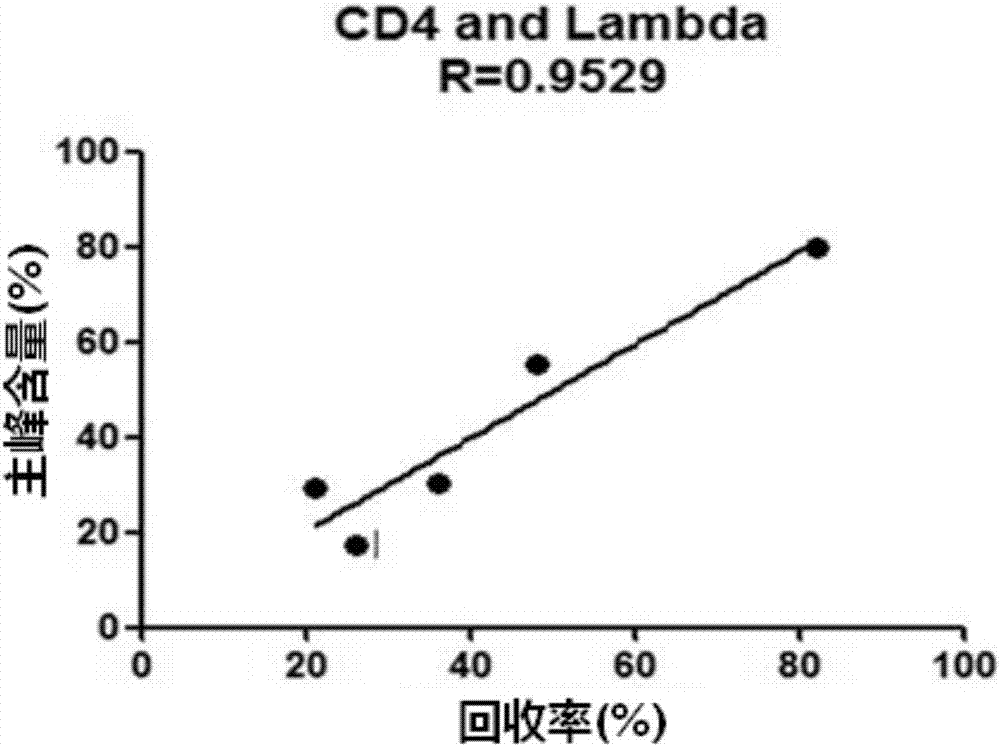
[0091] 3) Perform four-parameter fitting according to the ratio of Em650 / Em620 (Em665 / Em620*104) fluorescence and the concentration of the standard to obtain a standard curve, and calculate the concentration of the sample to be tested according to the standard curve. standard curve as figure 1 shown. figure 1 The middle abs...
Embodiment 2
[0093] Example 2, for detecting the integrity and biological activity of light chain and heavy chain:
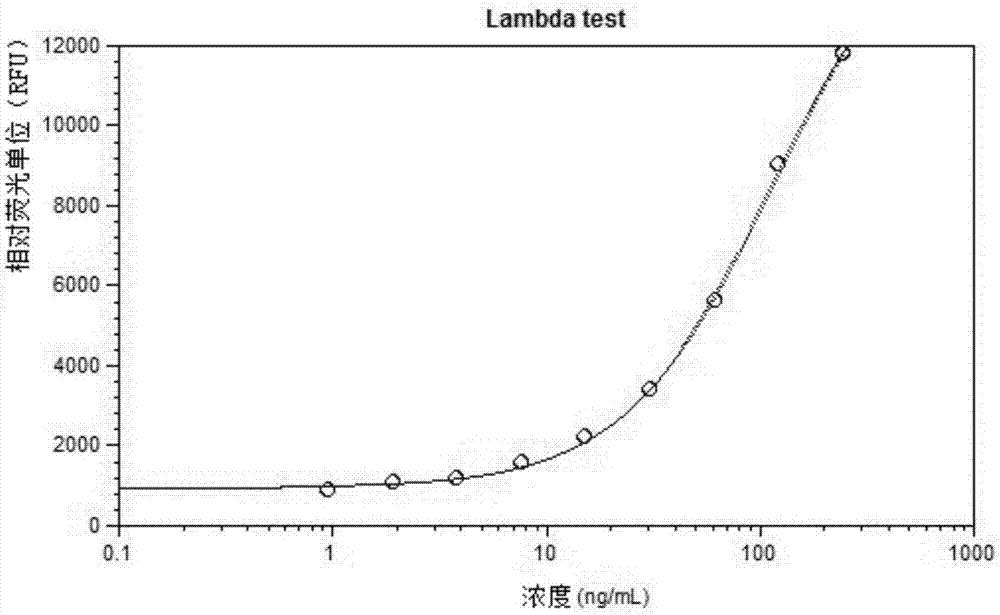
[0094] 1) Add cell supernatant, Anti-Lambda-Eu to 384 microwell plate #1 3+ cryptate (cryptate-coupled anti-Lambda light chain antibody), Anti-(h)Fc-d2 (d2-linked anti-human IgG Fc antibody), mix well. Incubate at room temperature for 2 hours or more or overnight with the target bispecific antibody as a standard.
[0095] 2) Add cell supernatant, Anti-(h)Fc-d2 (anti-human IgG Fc antibody linked to d2), Anti-his-Eu in 384 microwell plate 3+ cryptate (anti-His antibody conjugated to europium crypt), CD4 protein (his tag), mix well. Incubate at room temperature for 2 hours or more or overnight with the target bispecific antibody as a standard.
[0096] 3) Set the fluorescence wavelength: Excitation 313nm, Emission 665nm / 620nm, cutoff 630nm / 590nm, and read the fluorescence value.
[0097] 4) Perform four-parameter fitting according to the ratio of Em650 / Em620 (Em665 / Em620*...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com