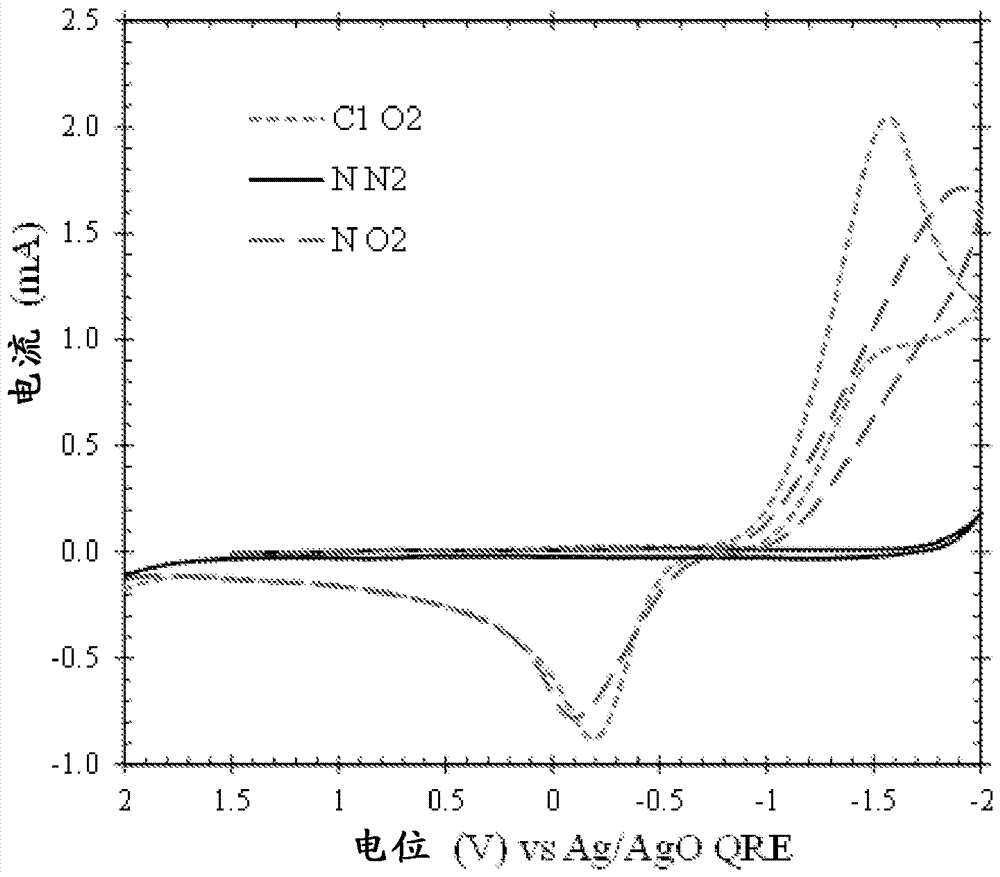
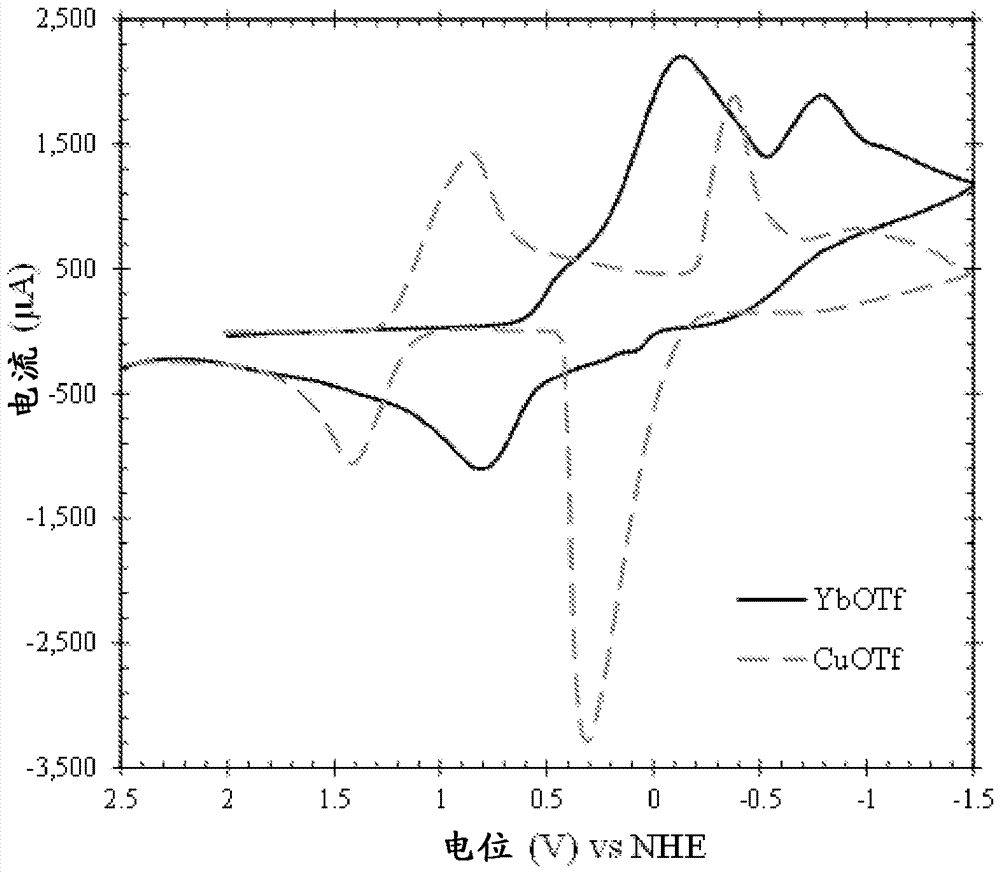
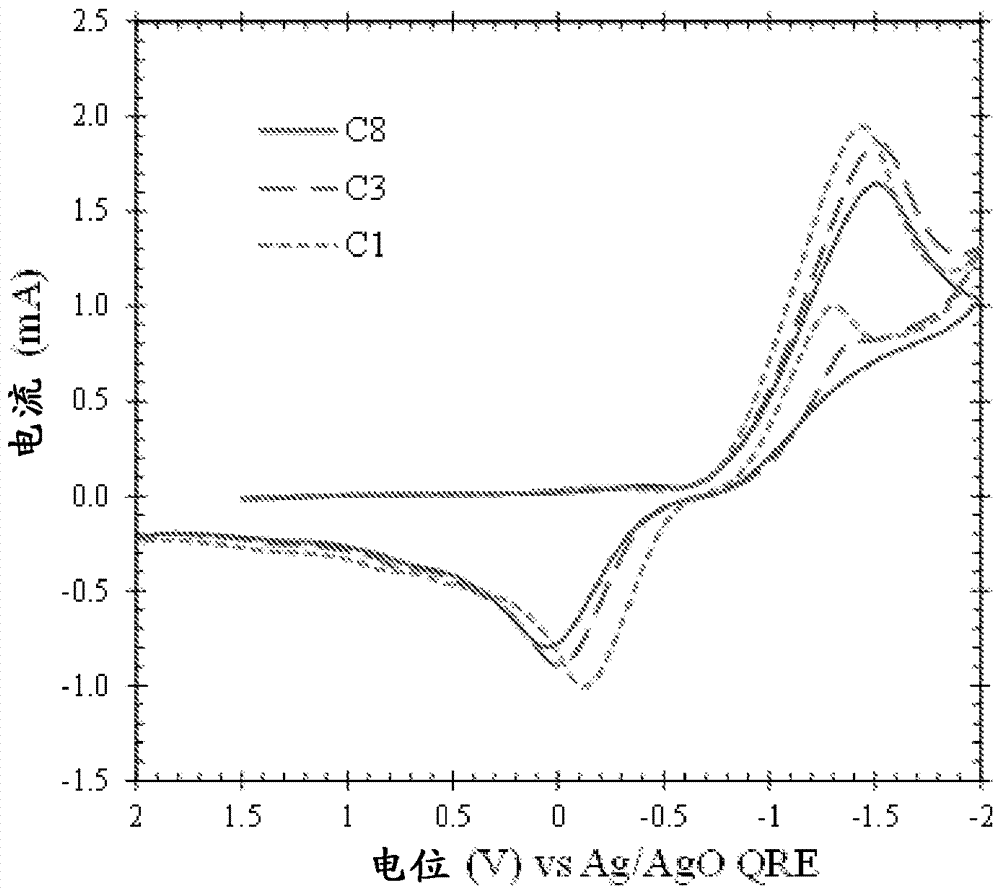
Lanthanide and actinide electrochemistry
A technology of lanthanides and actinides, which is applied in the field of electrochemistry of lanthanides and actinides, and can solve problems such as unrepeatable results and no modified electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0181] Example A : for TBABF in acetonitrile 4 The electrolyte was modified with a NAFION membrane (non-magnetic modification) for one working electrode, and the following scheme at a single working electrode allowed the separation of all lanthanides except Sm+Yb.
[0182]
Embodiment B
[0183] Example B : for TBABF in acetonitrile 4 One working electrode modified with NAFION membrane + magnetic modification in the electrolyte, the following scheme at a single working electrode allows separation of all lanthanides except Sm+Gd. (all separated at 30mV except step 2)
[0184]
Embodiment C
[0185] Example C : for TBABF in acetonitrile 4 Two working electrodes in the electrolyte, one with NAFION membrane (N) and one with NAFION membrane + magnetic modification (M), in the following scheme of two working electrodes (where one electrode is used at a time) allows separation of all.
[0186]
[0187] Using NAFION membranes with and without magnetic modification allowed separation of all five lanthanides.
[0188] These examples are not limiting or exclusive. They are possible scenarios based on the data in Table 1. Depending on the metal present, concentration and potential shifts available based on ligand, solvent and magnetic modification, other schemes may be more effective.
[0189] Separation may be able to be achieved by apparent potential shifts or by electron transfer kinetic constraints and chemical steps or both. Stronger magnets allow different potential shifts, allowing other separation schemes.
[0190] Larger offsets make separation more possibl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com