Methods for diagnosing cancer and determining the overall survival and disease-free survival of cancer patients
A technology for cancer and patients, applied in the field of kits that can be used in such methods, can solve problems such as inability to accurately predict the course of the disease, and achieve the effect of increasing the possibility of survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] GP88 Expression in Invasive Ductal Carcinoma
[0106] The present method is based on GP88 staining in formalin-fixed paraffin-embedded human breast lesions studied as clinicopathological variables. Cytoplasmic GP88 staining is observed in breast cancer and is almost always negative in benign breast epithelium. Tissue sections of 4–6 μm were prepared from 203 formalin-fixed paraffin-embedded biopsies. GP88 staining by immunohistochemical method using anti-human GP88 antibody, in normal tissues, benign lesions, ductal and lobular carcinomas (Table 1: DCIS: ductal carcinoma in situ; IDC: invasive carcinoma in situ; LCIS: lobular in situ Carcinoma; ILC: Infiltrating Lobular Carcinoma) the expression of GP88 was examined. In addition, a correlation study between GP88 expression in IDC and histological grade, proliferation index (Ki67), p53, ER and Her-2 expression was performed.
[0107] diagnosis N 0 1+ 2+ 3+ benign 26 25(96%) 1(4%) 0 0 DCIS...
Embodiment 2
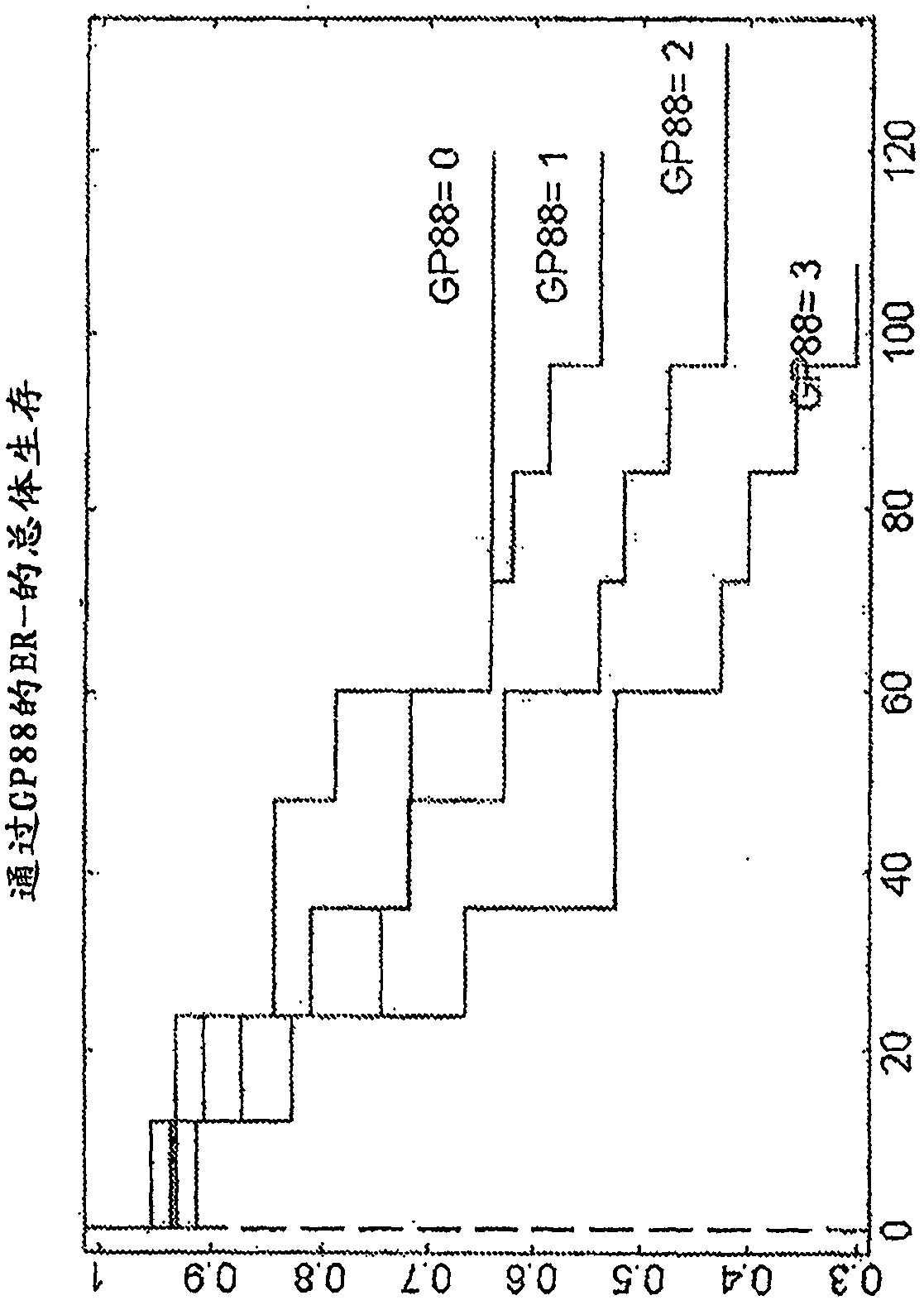
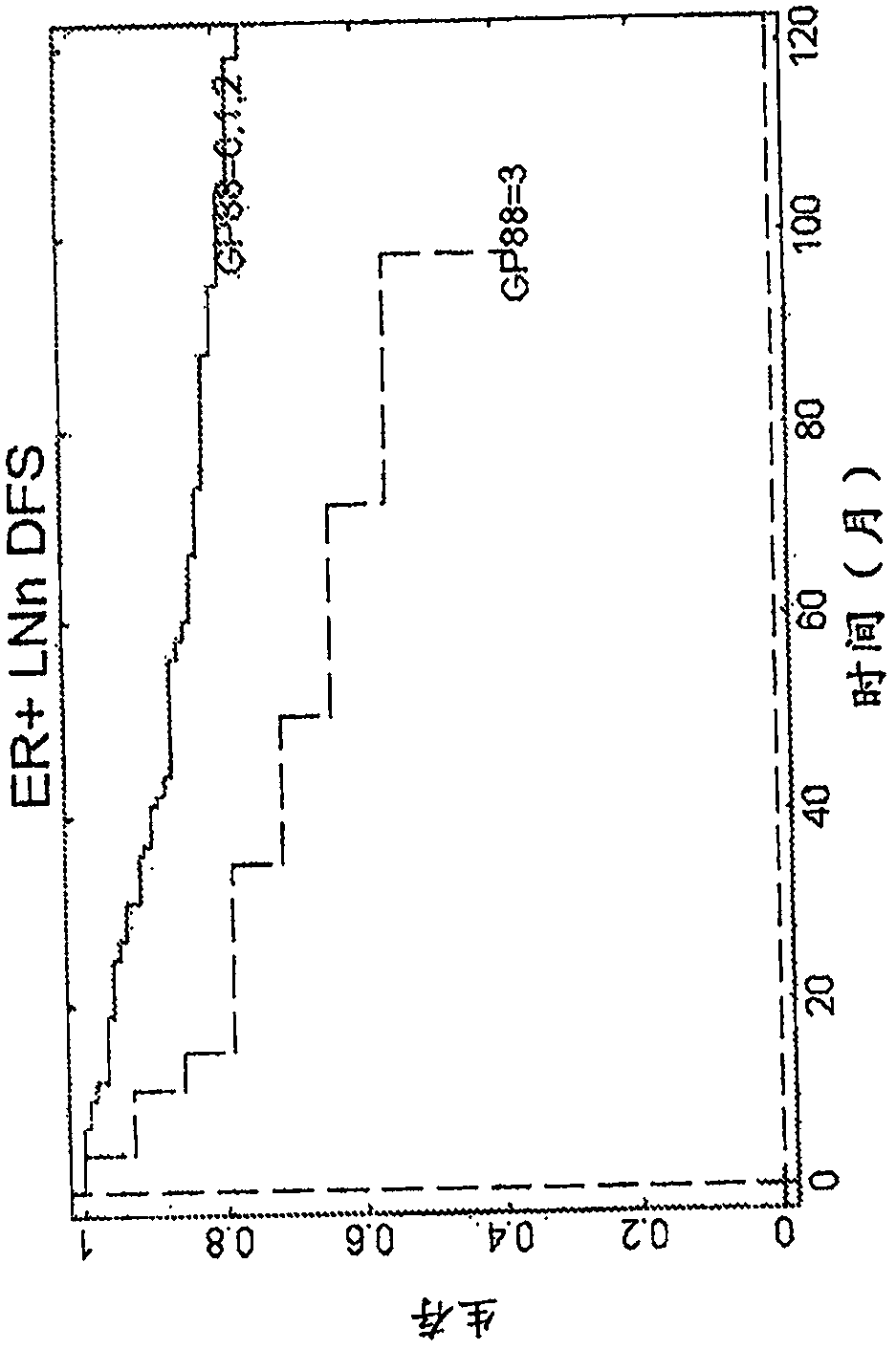
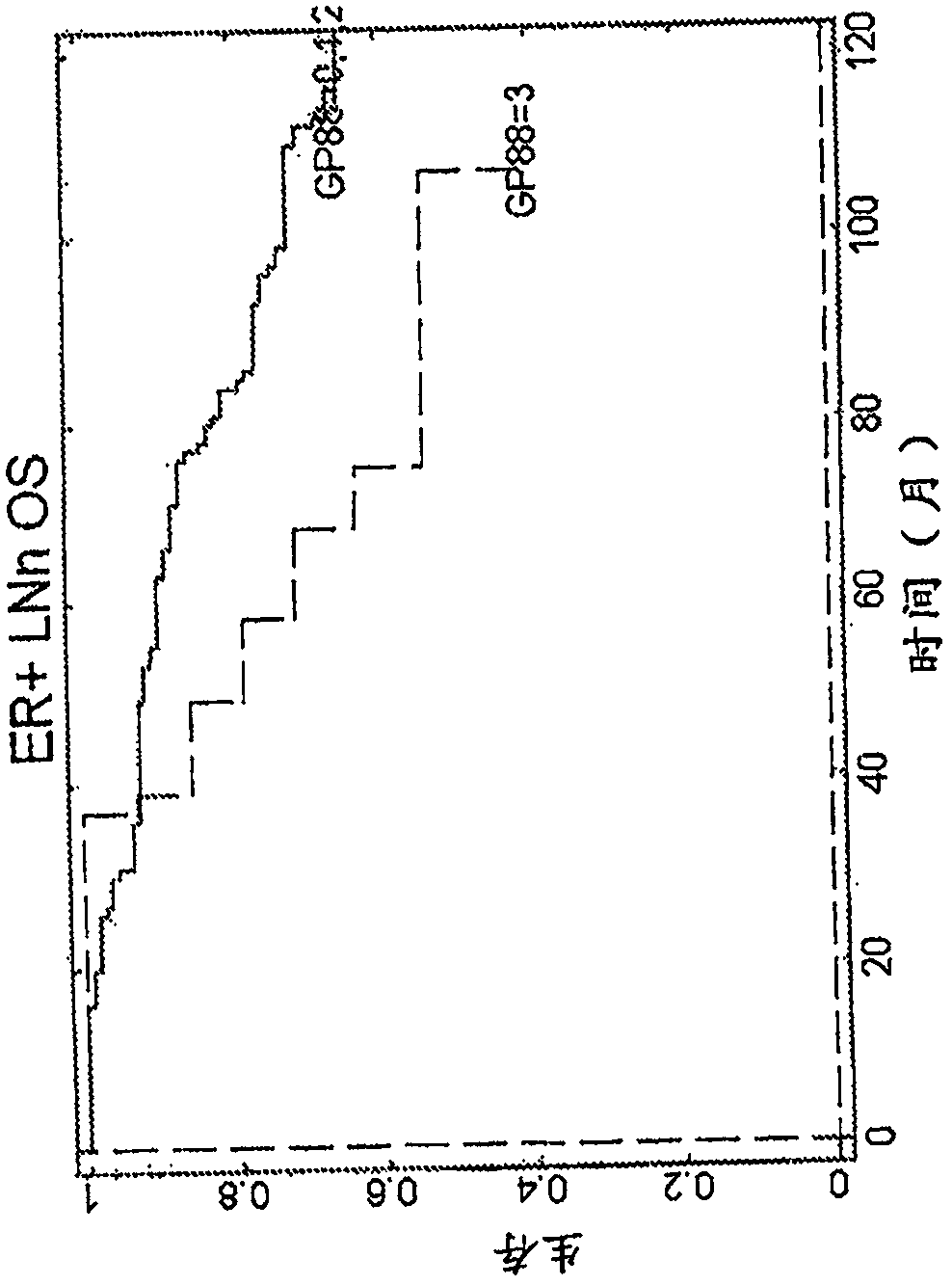
[0111] Analysis of GP88 expression level in breast cancer tissues of ER-, ER+ / LN- and ER+ / LN+ patients
[0112] Research design
[0113] The clinical study was performed on 389 breast cancer cases (see Table 2 for the characteristics of the subjects considered in this clinical study), specifically invasive ductal carcinoma tissue samples stored as formalin-fixed paraffin Embedded blocks, obtained from 3 tissue banks. Inclusion criteria were as follows: year and age at diagnosis, tumor characteristics: estrogen receptor (ER), progesterone receptor (PR), tumor grade, tumor size, nodal status, status at last follow-up, overall Survival (OS), relapse status, time to first relapse, treatment (ER+, LN0 tamoxifen). Cases meeting the inclusion criteria were pulled to prepare slides for the study.
[0114] Research methods
[0115] For each case, 4–6 micron tissue sections were freshly dissected from the histology laboratory at the bank processing site and mounted on positive...
Embodiment 3
[0135] Analysis of GP88 expression level in biological fluids obtained from breast cancer patients
[0136] The concentration of GP88 in human serum samples was determined in triplicate by enzyme-linked immunosorbent assay (ELISA). The recombinant GP88 was diluted in a solution of 30% glycerol+1% milk (milk)-PBS to prepare standard GP88 samples with concentrations of 0, 0.1, 0.25, 0.5, 1, 3, 10 and 20 ng / ml. 100 microwells on a microtiter plate were coated with 10 μg / ml of anti-human GP88 monoclonal antibody 6B3 (0.78 mg / ml 6B3 antibody dissolved in phosphate buffered saline (PBS)) and incubated overnight at 4°C. The wells were washed with PBS, and anti-human PCDGF polyclonal (IgG fraction) was added to each well at a concentration of 3 µg / ml, and incubated at 37°C for 1.5 hours. Wells were washed with PBS, and a detection antibody (horseradish peroxidase (HRP)-goat-rabbit-IgG) was added to each well. TMB (substrate) was added and samples were incubated for 1 hour. The op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com