A kind of preparation method of allene compound containing ferrocene and phosphate group
A dimethyl phosphate allene and phosphate-based technology, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems that there are no reports on the synthesis of allene compounds, and achieve high yield and fast synthesis rate , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of 1-phenyl-3-ferrocenyl-1-phosphate dimethyl allene
[0025] Into a 25 mL glass reaction vial, add 58 mg (ie 0.26 mmol) dimethyl benzyl phosphate diazo, 42 mg (ie 0.2 mmol) ferroceneacetylene, 7.6 mg (ie 0.04 mmol) cuprous iodide and 40 mg (that is, 0.4 mmol) triethylamine, then add 2 mL of dioxane, under the protection of nitrogen, the temperature is 70 ° C, the reaction is stirred for 1 hour, after the reaction is concentrated, the volume ratio of petroleum ether: ethyl acetate is 1:1 As eluent, purified by silica gel column chromatography to obtain 1-phenyl-3-ferrocenyl-1-phosphate dimethyl allene, its structure is shown in the following formula:
[0026]
[0027] The compound is an orange-yellow liquid with a yield of 66%, and its NMR data are as follows:
[0028] 1 H NMR (500 MHz, CDCl 3 ) δ 3.80 (t, J = 5.0 Hz, 3H), 3.83 (t, J = 5.0Hz, 3H), 4.21 (s, 5H), 4.26 (d, J = 1.5 Hz, 2H), 4.33 (d, J = 12 Hz, 2H),6.51 (d, J = 12.5 Hz, 1H), 7.29 (d, J = 7 H...
Embodiment 2
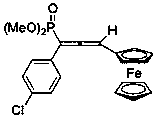
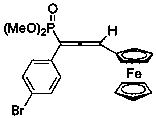
[0030] Synthesis of 1-(4-chlorophenyl)-3-ferrocenyl-1-phosphate dimethyl allene
[0031] Add 68 mg (ie 0.26 mmol) dimethyl p-chlorobenzyldiazophosphate, 42 mg (ie 0.2 mmol) ferroceneacetylene, 7.6 mg (ie 0.04 mmol) cuprous iodide to a 25 mL glass reaction vial and 40 mg (that is, 0.4 mmol) triethylamine, and then add 2 mL of dioxane, under the protection of nitrogen, the temperature is 70 ° C, react and stir for 2 hours, after the reaction, concentrate, and the volume ratio of petroleum ether: ethyl acetate is 1 :1 can be used as eluent and purified by silica gel column chromatography to obtain 1-(4-chlorophenyl)-3-ferrocenyl-1-phosphate dimethyl allene, and its structure is shown in the following formula:
[0032]
[0033] The compound is an orange-yellow liquid with a yield of 57%, and its NMR data are as follows:
[0034] 1 H NMR (500 MHz, CDCl 3 ) δ 3.80 (t, J = 4.0 Hz, 3H), 3.82 (t, J = 2.0Hz, 3H), 4.21 (s, 5H), 4.27 (t, J = 1.5Hz, 2H), 4.30-4.33 (m , 2H), 6.52 (d,...
Embodiment 3
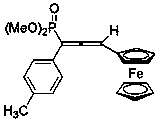
[0036] Synthesis of 1-(4-methylphenyl)-3-ferrocenyl-1-phosphate dimethyl allene
[0037] Add 63 mg (ie 0.26 mmol) dimethyl benzyl phosphate diazonium, 42 mg (ie 0.2 mmol) ferroceneacetylene, 7.6 mg (ie 0.04 mmol) cuprous iodide to a 25 mL glass reaction vial and 40 mg (that is, 0.4 mmol) triethylamine, then add 2 mL of dioxane, under the protection of nitrogen, the temperature is 80 ° C and stirred for 2 hours, concentrated after the reaction, and the volume ratio of petroleum ether: ethyl acetate is 1 :1 can be used as eluent and purified by silica gel column chromatography to obtain 1-(4-methylphenyl)-3-ferrocenyl-1-phosphate dimethyl allene, and its structure is shown in the following formula:
[0038]
[0039] The compound is an orange-yellow liquid with a yield of 83%, and its NMR data are as follows:
[0040] 1 H NMR (500 MHz, CDCl 3 ) δ 2.34 (s, 3H), 3.78 (t, J = 4.0 Hz, 3H), 3.82(t, J = 5.75, 3H), 4.21 (s, 5H), 4.25 (t, J = 2.0 Hz, 2H) , 4.30-4.33 (m, 2H),6.48 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com