Compound imidazoline corrosion inhibitor, and preparation method thereof
A technology for compounding imidazoline and imidazoline, applied in the direction of organic chemistry, etc., to achieve the effects of good water solubility, inhibition of metal corrosion, and good corrosion inhibition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] step one
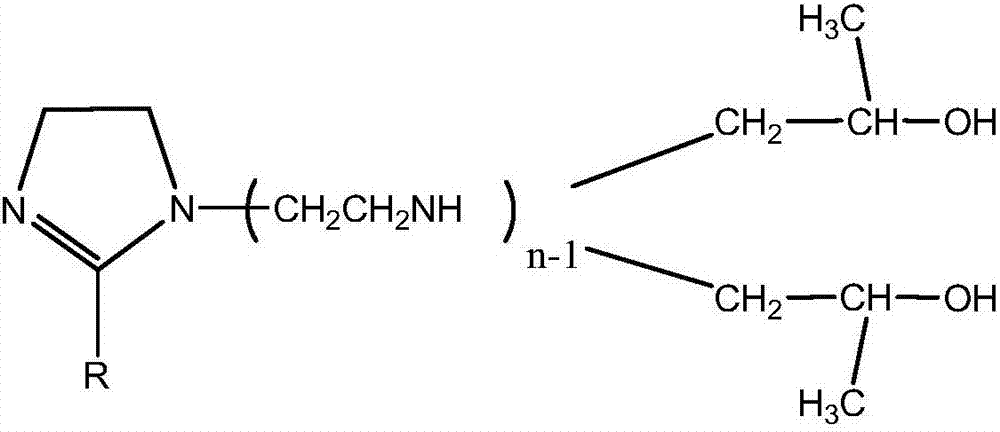
[0030] Add triethylenetetramine and octadecenoic acid in a molar ratio of 1.2:1 into a high-temperature reaction kettle equipped with a stirring, condenser, water separator and thermometer, add 60 g of water-carrying agent xylene, and pass through N2 Exclude the air in the system, start stirring and gradually raise the temperature, control the temperature at 135°C for 2 hours, then continue to raise the temperature to 235°C for 3 hours, until the xylene and excess materials are distilled off, and the imidazoline compound I is obtained.
[0031] step two
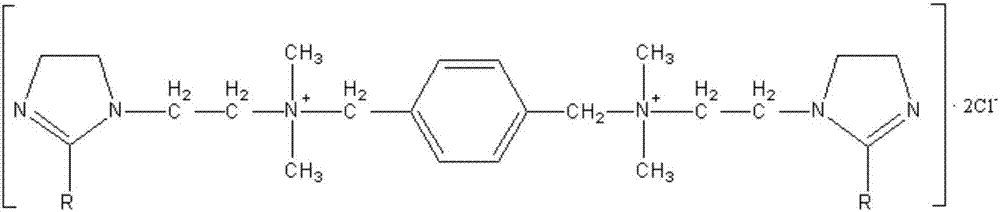
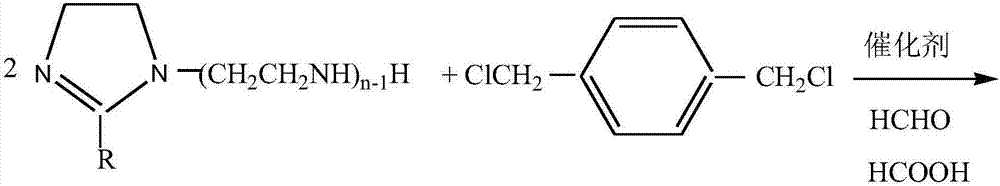
[0032] Add the imidazoline compound I and 1,4-bis(chloromethyl)benzene obtained in step 1 into a high-temperature reaction kettle equipped with stirring, condenser, water separator and thermometer according to the molar ratio of 2.1:1, Add an appropriate amount of sodium methoxide as a catalyst, pass N2 to remove the air in the system, start stirring and gradually increase the temperature, control the tempe...
Embodiment 2
[0037] step one
[0038] Add diethylenetriamine and lauric acid in a molar ratio of 1.15:1 into a high-temperature reaction kettle equipped with a stirring, condenser, water separator and thermometer, add 60 g of water-carrying agent xylene, and pass N 2 Exclude the air in the system, start stirring and gradually heat up, control the temperature at 130-135°C for 2 hours, then continue to heat up to 235°C for 2-3 hours, until the xylene and excess materials are evaporated to obtain imidazoline Compound I.
[0039] step two
[0040] Add the imidazoline compound I and 1,4-bis(chloromethyl)benzene obtained in step 1 into a high-temperature reaction kettle equipped with stirring, condenser, water separator and thermometer according to the molar ratio of 2.1:1, Add an appropriate amount of sodium methoxide as a catalyst, pass N 2 Exclude the air in the system, start stirring and gradually raise the temperature, control the temperature at 135-140°C for 2 hours to obtain imidazolin...
Embodiment 3
[0046] step one
[0047] Add tetraethylenepentamine and octadecenoic acid in a molar ratio of 1.15:1 into a high-temperature reaction kettle equipped with a stirring, condenser, water separator and thermometer, add 60 g of water-carrying agent xylene, and pass N 2 Exclude the air in the system, start stirring and gradually heat up, control the temperature at 130-135°C for 2 hours, then continue to heat up to 235°C for 2-3 hours, until the xylene and excess materials are evaporated to obtain imidazoline Compound I.
[0048] step two
[0049] Add the imidazoline compound I and 1,4-bis(chloromethyl)benzene obtained in step 1 into a high-temperature reaction kettle equipped with stirring, condenser, water separator and thermometer according to the molar ratio of 2.1:1, Add an appropriate amount of sodium methoxide as a catalyst, pass N 2 Exclude the air in the system, start stirring and gradually raise the temperature, control the temperature at 135-140°C for 2 hours to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com