Application of schisandrin B in drug preparation
A technology of schisandrin and medicine, which is applied in the field of medicine, can solve the problems that the effect of diabetic complications has not been reported, and the direct anti-inflammatory target of schisandrin is not clear, and achieve the effect of high-efficiency treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 5
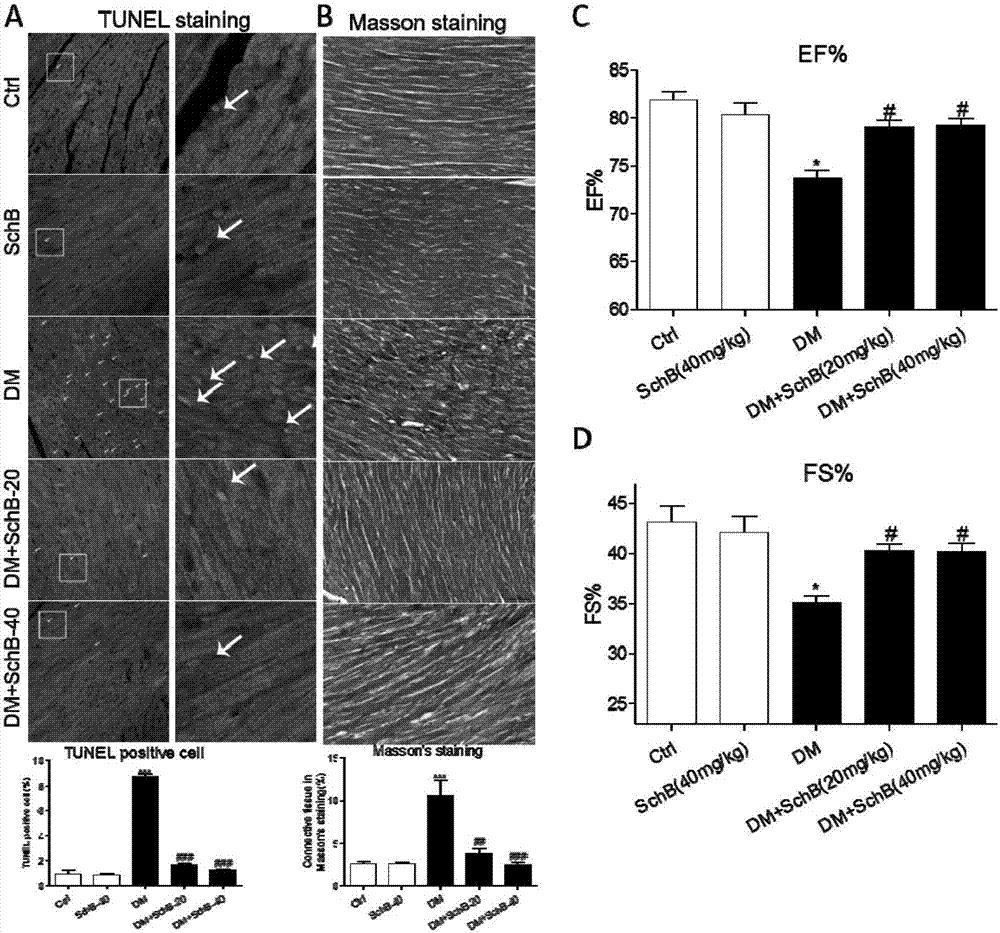
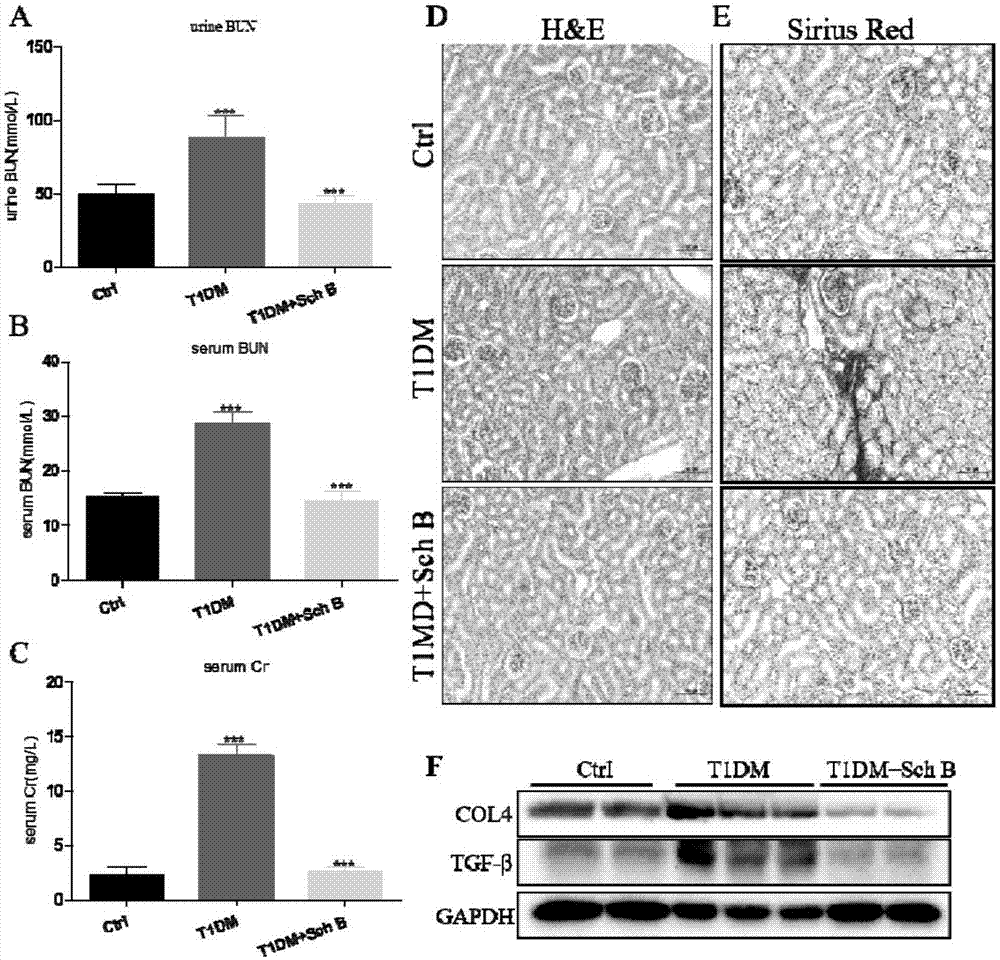
[0023] Example 1 Effect of Schizandrin B on Organ Complications in STZ-Induced Type 1 Diabetic Mice
[0024] Male C57BL / 6 mice were obtained from the Animal Experiment Center of Wenzhou Medical College. Mice were housed in a thermostated 12-12h diurnal animal room with standard rodent chow and water. Animals spend at least one week acclimating to the environment before the start of the experiment. All protocols involving the use of animals were approved by the Animal Policy and Welfare Committee of Wenzhou Medical College (approval document: 2009 / APWC / 0031). The Schizandrin B used in the experiment is a water-soluble dosage form. The pH of the solution was 7.36 and filtered through a 0.22 microporous membrane. Male C57BL / 6 mice aged 8-10 weeks were randomly divided into 6 groups: normal control group, Schisandrin B treatment group (Sch B), diabetes group (DM), diabetes group combined with Schizandrin B (20, 40 mg / kg / d two doses) treatment group, 8 rats in each group. Low...
Embodiment 2 5
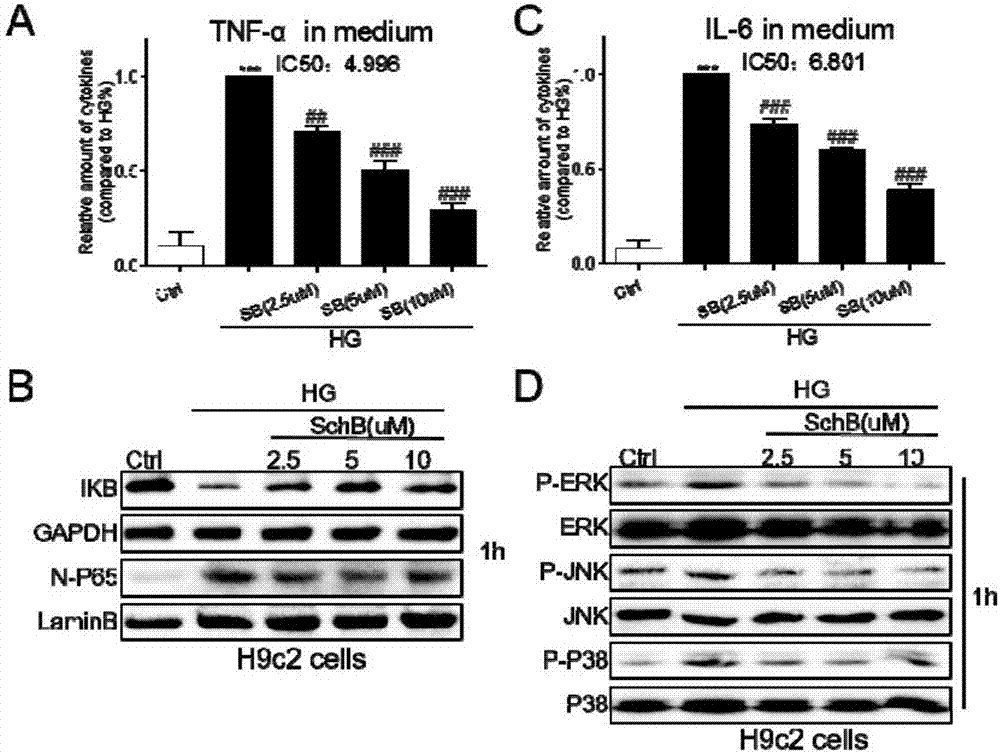
[0029] Example 2 Schizandrin B can alleviate the inflammatory response of cardiomyocytes induced by HG
[0030] The in vitro anti-inflammatory activity of Schisandrin B was tested by using the method of inhibiting the release of inflammatory factors (TNF-α and IL-6) in HG-stimulated H9c2 and rat primary cardiomyocytes. The specific method is as follows: 1.2×10 ^6 H9c2 or rat primary cardiomyocytes were cultured with DMEM medium at 37°C, the medium was renewed after 24 hours, and the tested Schisandrin B (final concentration was 2.5, 5 and 10 μM) was added for pretreatment for 1 hour, and then treated with high Sugar (HG 33mM glucose) was continued to be treated for 24 hours, and the culture medium was collected to detect TNF-α and IL-6 content by ELISA method; the cells were collected to detect the total protein concentration, and the ELISA results were divided by the corresponding total protein concentration for calibration, and compared with HG The content of TNF-α and IL-6 ...
Embodiment 3 5
[0034] Example 3 Schizandrin B specifically inhibits the complexation of TLR4 and Myd88 protein in the LPS-induced inflammatory signaling pathway
[0035] In the canonical LPS-stimulated inflammatory signaling pathway, TLR4 is the main receptor of LPS. However, the combination of TLR4 and LPS requires the participation of myeloid differentiation protein 2 (MD2) to form a LPS-TLR4-MD2 complex, which further recruits downstream intracellular adapter proteins, such as: Myd88-dependent inflammatory factor transcription pathway and TRIF-dependent interferon-beta (IFN-beta) transcription pathway. Among them, the complex of MD2 and TLR4 and the recruitment of downstream adapter protein Myd88 by TLR4 are important signs of the activation of this inflammatory pathway. We used co-immunoprecipitation experiments to detect the inhibitory activity of Schizandrin B on these two protein complexes in vitro. For experimental data, see Figure 4 . Experiments showed that schisandrin B inhibi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com