Periplaneta americana peptide C with anti-hepatoma activity and application of periplaneta americana peptide C
A technology of anti-liver cancer and cockroach peptide, which is applied in the field of medicine, can solve problems such as hypersensitivity, failure to meet the needs of the pharmaceutical market, and lower drug efficacy, and achieve the effect of small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] American cockroach peptide C exists in the medicinal materials of Periplaneta americana. The extraction method of the American cockroach peptide C comprises the following steps: after extracting the Periplaneta americana medicinal material with a solvent, and then repeatedly extracting and separating by macroporous resin adsorption, dextran gel column chromatography, high performance liquid chromatography, etc., A sample containing peptidic acid C was obtained.
[0035] The polypeptide sequence Asp-Leu-Asn-Asn-Ser-Arg-Lys of American cockroach peptide C was entrusted to Sangon Bioengineering (Shanghai) Co., Ltd. for synthesis. The physical and chemical properties of peptide C are as follows:
[0036] Number of amino acid residues:
7
1 character:
DLNNSRK
3 characters:
Asp-Leu-Asn-Asn-Ser-Arg-Lys
Molecular weight:
845.91g / mol
10.1
Net charge at PH=7:
1.0
Average Hydrophilicity:
...
Embodiment 2
[0041] 1. Cell culture of human liver cancer HepG2 cell line
[0042] Human liver cancer HepG2 cell line adopts high-glucose DMEM complete medium containing 10% fetal bovine serum, placed in 5% CO 2 , Cultured in a 37°C incubator, and the cells in the logarithmic growth phase were taken for experiments.
[0043] 2. MTT method to detect the survival rate of human liver cancer cell HepG2 cells after treatment
[0044] HepG2 cell seed plate (1×10 5 mL -1, 100 μL) were inoculated in a 96-well plate and cultured for 24 h. Prepare the American cockroach peptide C solutions with concentrations of 6.25, 12.5, 25, 50, 100, and 150 μg / mL, intervene with the above-mentioned 6 concentrations of Periplaneta americana peptide solutions, set 6 replicate wells for each concentration, and set up 6 replicate wells for each concentration. 200 μL, set thalidomide (concentration: 100 μg / mL, 200 μg / mL) positive control group and blank control group at the same time, continue to culture for 24 h...
Embodiment 3
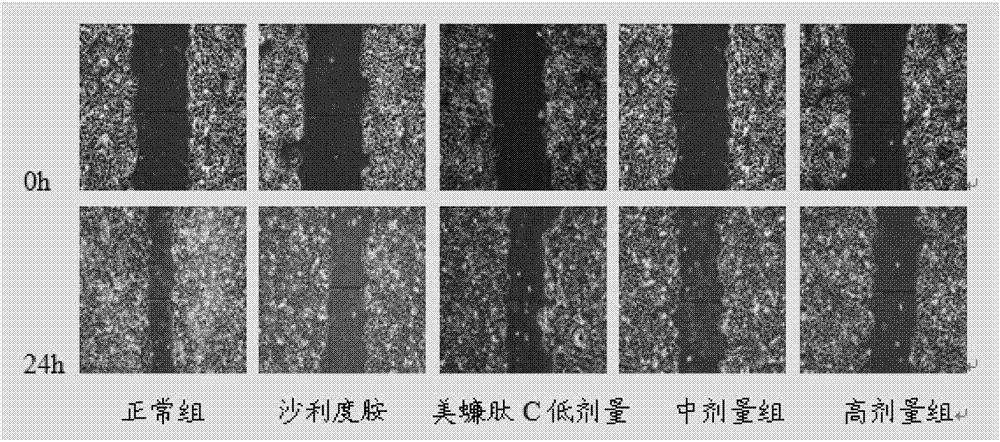
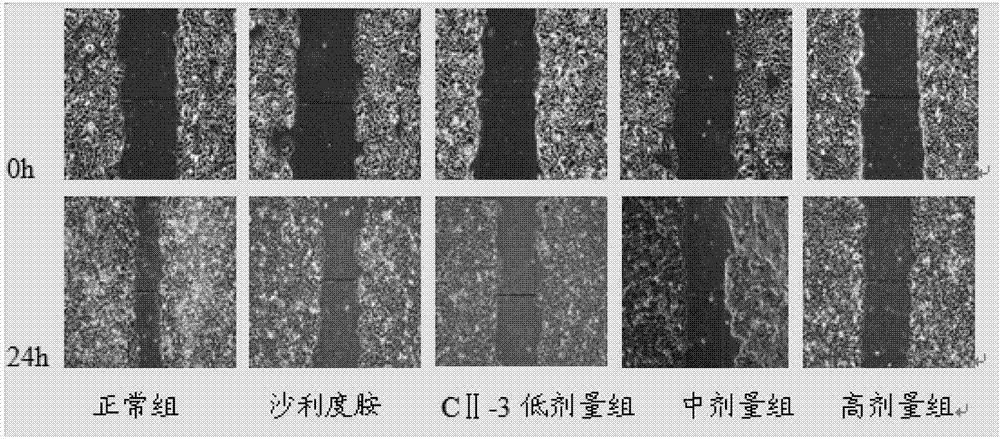
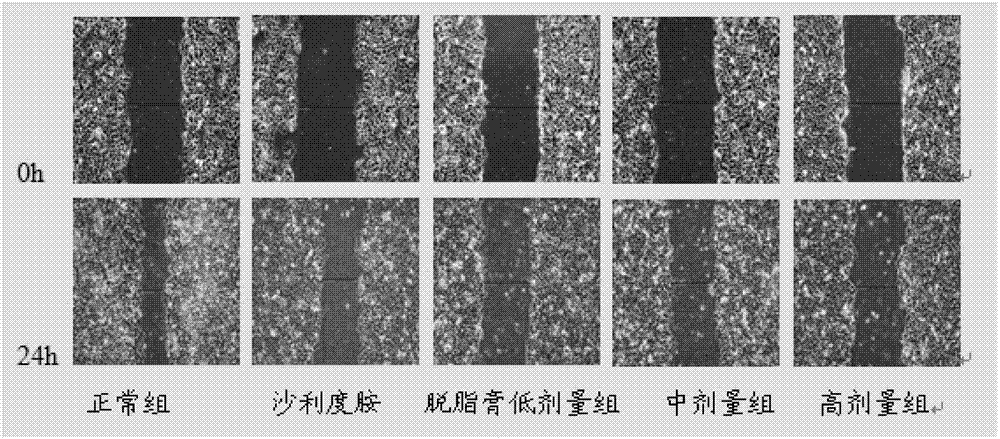
[0057] Inhibition of Migration of HepG2 Cells by Peptide C in Cell Scratch Test
[0058] Experimental grouping: blank control group, thalidomide control group (concentration of 100 μg / mL, 200 μg / mL), meicockatide C concentration set to low, medium and high dose groups (25 μg / mL, 50 μg / mL, 100 μg / mL mL, 150 μg / mL).
[0059] HepG2 cells were routinely digested at a density of 2×10 5 mL -1 Inoculate in a 6-well plate, 2 mL per well. After culturing for 24 hours, use a 200 μL pipette tip to draw a straight line at the bottom of the 6-well plate, wash with PBS 2 to 3 times, and add drugs according to the experimental groups. Take pictures of the scratches at 0h and 24h respectively, and measure the width of the scratches. The above experiment was repeated three times, and the average value was taken to calculate the scratch healing rate. Scratch healing rate=(0h scratch width-24h scratch width) / 0h scratch width×100%.
[0060] SPSS 17.0 statistical software and OriginPro 8.6 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com