Application of isorhodanic ester type compound to prevention and/or treatment of hyperbilirubinemia
A technology for hyperbilirubinemia and compounds, applied in the direction of silicon compound active ingredients, ester active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1 The study of reducing total bilirubin activity and blood lipids in patients with benign prostatic hyperplasia
[0152] Experimental purpose: To study the clinical efficacy and safety of oral phenethyl isothiocyanate capsules in the treatment of benign prostatic hyperplasia (BPH).
[0153] Experimental design: A multi-center, randomized, double-blind, placebo-controlled design was used to preliminarily evaluate the safety and effectiveness of phenylethyl isothiocyanate capsules in the treatment of BPH patients.
[0154] Subjects who met the inclusion and exclusion criteria were randomly assigned to any of the following four groups according to the ratio of 1:1:1:1: Group A: phenylethyl isothiocyanate 10 mg / time, 3 times a day group D: phenylethyl isothiocyanate 20mg / time, 3 times a day; group B: phenylethyl isothiocyanate 30mg / time, 3 times a day; group C: phenethyl isothiocyanate Cyanate placebo 1 capsule / time, 3 times a day. Oral administration, the course ...
Embodiment 2
[0198] Example 2 Auxiliary detection of total bilirubin and blood lipids
[0199] Using the same method as in Example 1, 24 volunteers with benign prostatic hyperplasia and hyperbilirubinemia were recruited from the medical research colleagues. In the same manner as in Example 1, subjects who met the inclusion and exclusion criteria were randomly assigned to any of the following four groups in a ratio of 1:1:1:1: Administration group (Group A): Benzene Ethyl isothiocyanate 10mg / time, 3 times a day; administration group (group D): phenylethyl isothiocyanate 20mg / time, 3 times a day; administration group (group B): benzene Ethyl isothiocyanate 30mg / time, 3 times a day; placebo group (group C): phenethyl isothiocyanate placebo 1 capsule / time, 3 times a day. Oral administration, the course of treatment is 24 weeks.
[0200] Evaluate the effectiveness and safety of the experiment. Effectiveness: ①Benign prostatic hyperplasia: IPSS, QOL, prostate volume, maximum urinary flow rate...
Embodiment 3
[0202] Example 3 Auxiliary detection of total bilirubin
[0203] The method is the same as in Example 2, the difference is that 8 volunteers who only suffer from hyperbilirubinemia are recruited from the medical research colleagues.
[0204] In the same manner as in Example 2, the subjects were randomly assigned to any one of the following four groups in a ratio of 1:1:1:1: administration group (group A): phenylethyl isothiocyanate Ester 10mg / time, 3 times a day; administration group (group D): phenylethyl isothiocyanate 20mg / time, 3 times a day; administration group (group B): phenethyl isothiocyanate Ester 30mg / time, 3 times a day; placebo group (Group C): phenethyl isothiocyanate placebo 1 capsule / time, 3 times a day. Oral administration, the course of treatment is 6 weeks.
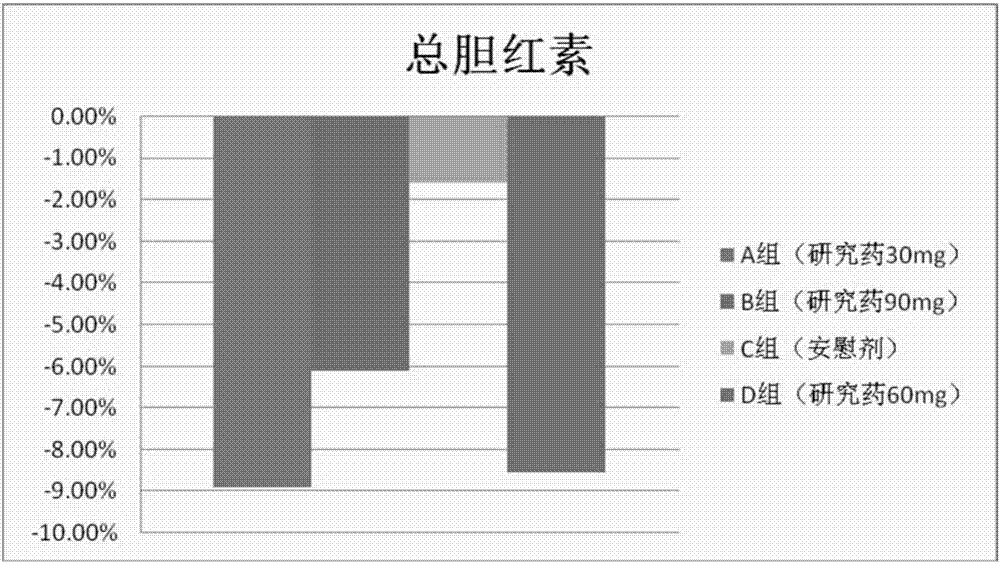
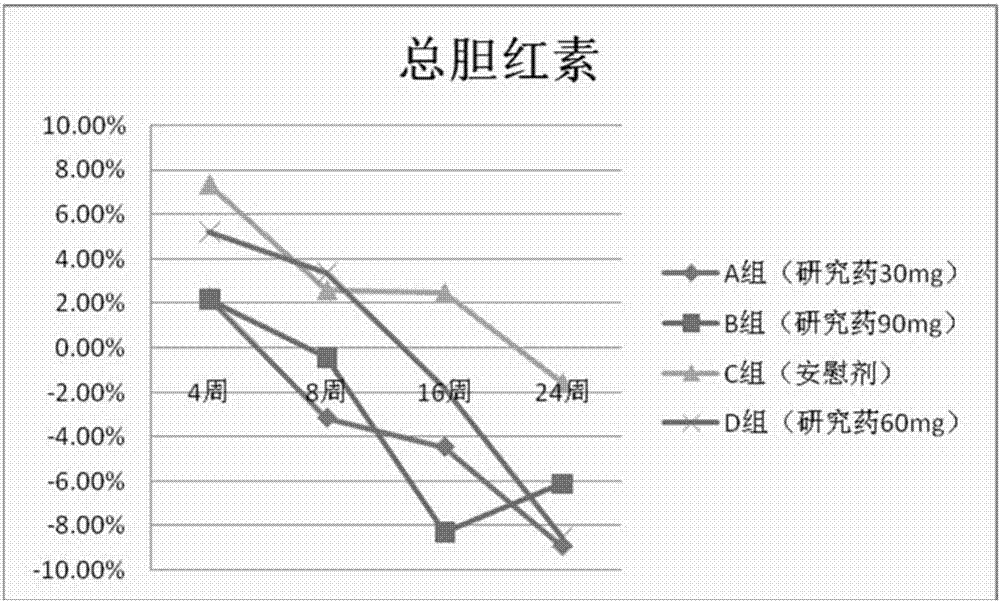
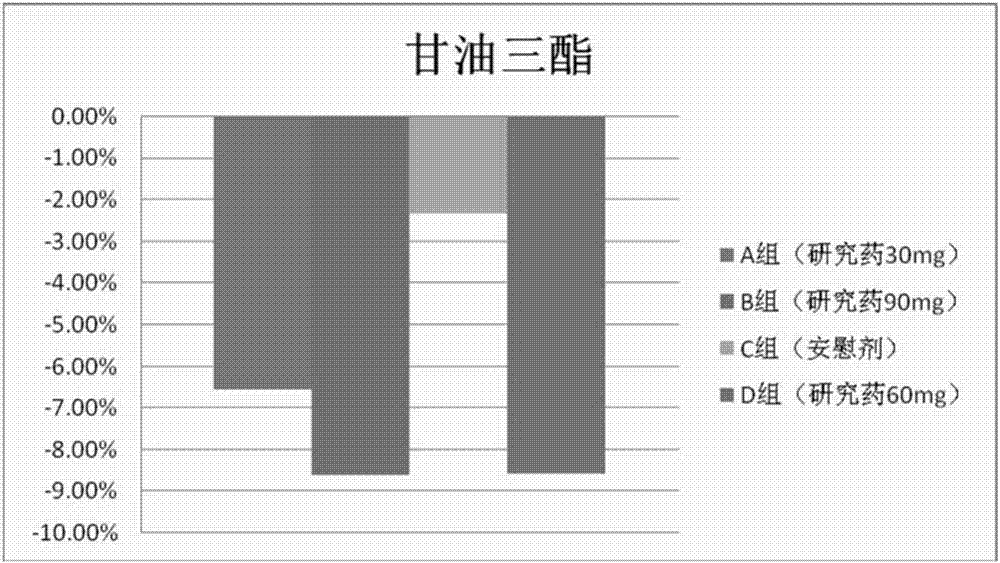
[0205] The results show that after the volunteers take the isothiocyanate compound (such as phenylethyl isothiocyanate) of the present invention, the total bilirubin level is significantly decreased,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com