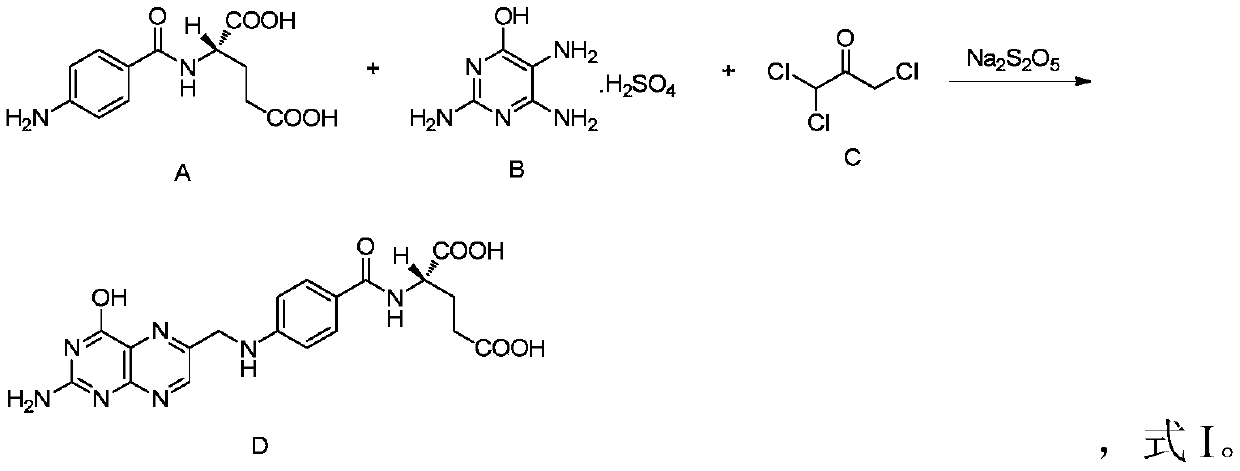
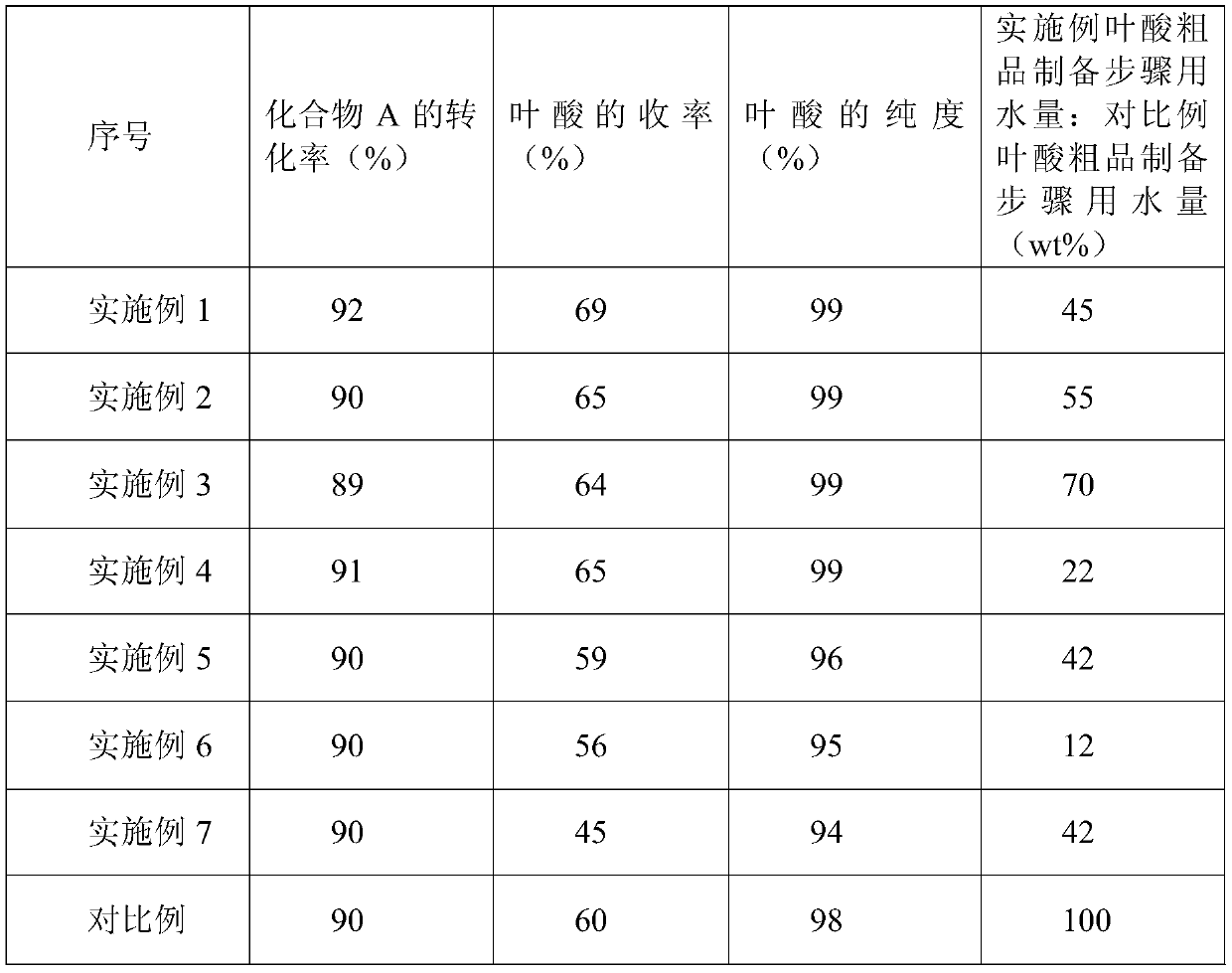
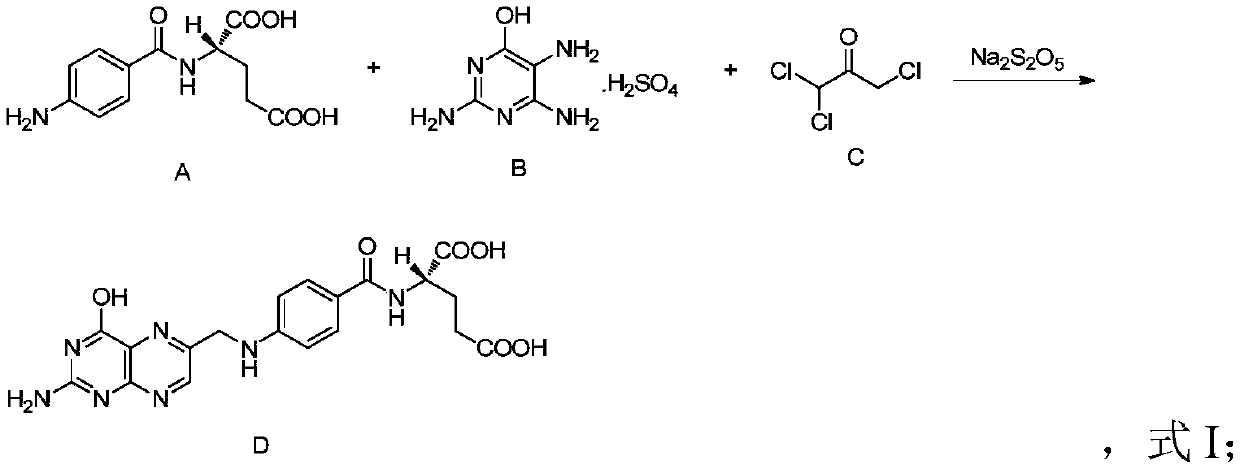
A kind of production method of folic acid
A production method, folic acid technology, applied in the direction of organic chemistry, etc., can solve the problems of complex process and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this embodiment, in the crude product synthesis step, the relationship of the total amount of materials is as follows:
[0037] N-(p-aminobenzoyl)-L-glutamic acid: 26.6kg;
[0038] 2,4,5-triamino-6-hydroxypyrimidine sulfate: 23.9kg;
[0039] Trichloroacetone crystallization (purity 92%): 25.6kg;
[0040] Sodium metabisulfite: 9.5kg;
[0041] 700L of water;
[0042] Wherein, the material molar ratio relationship is as follows: N-(p-aminobenzoyl)-L-glutamic acid: 2,4,5-triamino-6-hydroxypyrimidine sulfate: trichloroacetone crystallization: sodium pyrosulfite=1: 1:1.6:0.5;
[0043] The volume ratio of the total molar weight of N-(p-aminobenzoyl)-L-glutamic acid to water is 0.14mol / L;
[0044] Materials N-(p-aminobenzoyl)-L-glutamic acid, 2,4,5-triamino-6-hydroxypyrimidine sulfate, trichloroacetone crystals and sodium metabisulfite were added in two batches, the first batch Add 1 / 2 of the total amount of corresponding materials for the first time, and add the remai...
Embodiment 2
[0056] In this embodiment, in the crude product synthesis step, the material relationship is as follows:
[0057] The molar ratio of materials is as follows: N-(p-aminobenzoyl)-L-glutamic acid: 2,4,5-triamino-6-hydroxypyrimidine sulfate: trichloroacetone crystallization: sodium pyrosulfite=1:1.3: 2:0.5;
[0058] The volume ratio of the total molar weight of N-(p-aminobenzoyl)-L-glutamic acid to water is 0.1mol / L;
[0059] Among them, the materials N-(p-aminobenzoyl)-L-glutamic acid, 2,4,5-triamino-6-hydroxypyrimidine sulfate and sodium pyrosulfite are added in two batches. Add 2 / 3 of the total amount of corresponding materials for the first time, and add the remaining materials in the second batch.
[0060] After the first batch of material is added, adjust the pH value between 3.0-3.5 with 10wt% sodium carbonate solution, and control the reaction system pH between 3.0-3.5 in the reaction process, LC-MS detects the above-mentioned system, when N- Add the second batch of mat...
Embodiment 3
[0065] In this embodiment, in the crude product synthesis step, the material relationship is as follows:
[0066] The molar ratio of the materials is as follows: N-(p-aminobenzoyl)-L-glutamic acid: 2,4,5-triamino-6-hydroxypyrimidine sulfate: trichloroacetone crystallization: sodium metabisulfite=1:2: 3:0.8;
[0067] The volume ratio of the total molar weight of N-(p-aminobenzoyl)-L-glutamic acid to water is 0.08mol / L;
[0068] Among them, the materials N-(p-aminobenzoyl)-L-glutamic acid, 2,4,5-triamino-6-hydroxypyrimidine sulfate and sodium metabisulfite are added in three batches. Add 1 / 2 of the total amount of corresponding materials for the first time, add 1 / 2 of the remaining materials for the second batch, and add the remaining materials for the third batch.
[0069] The first batch of reaction temperature is 35°C, after the first batch of materials are added, adjust the pH value to between 3.0-3.5 with 10wt% sodium carbonate solution, and control the pH of the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com