Synthetic method of 2-(trimethylsilyl)phenyl trifluoromethane sulfonate
A technology of phenyl trifluoromethane sulfonate and trimethylsilyl is applied in the field of synthesis of pharmaceutical intermediates and chemical materials, and can solve the problems of difficult operation, harsh reaction conditions, and high cost of raw materials, and achieves reaction equipment. The effect of simple requirements, mild reaction conditions, and easy control of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
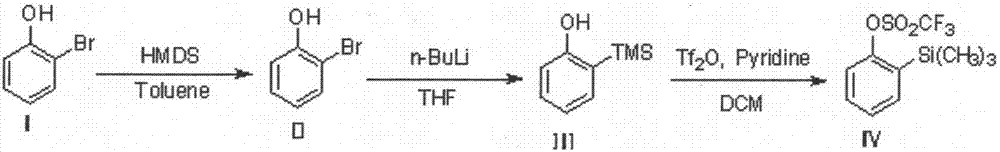
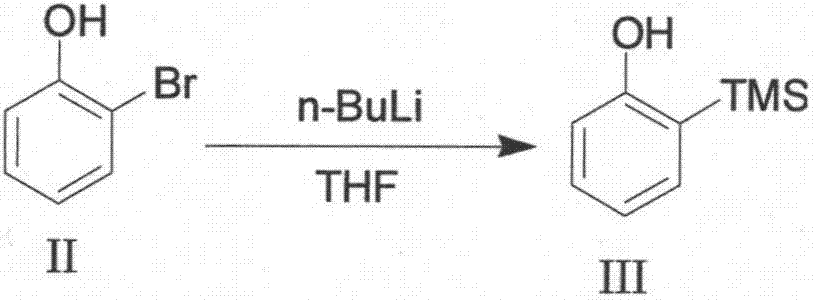
[0022] Process method for synthesizing 2-(trimethylsilyl) phenyl trifluoromethanesulfonate
[0023] The chemical equation of the synthesis process of the present invention is as follows:
[0024]
[0025] The specific implementation steps are:
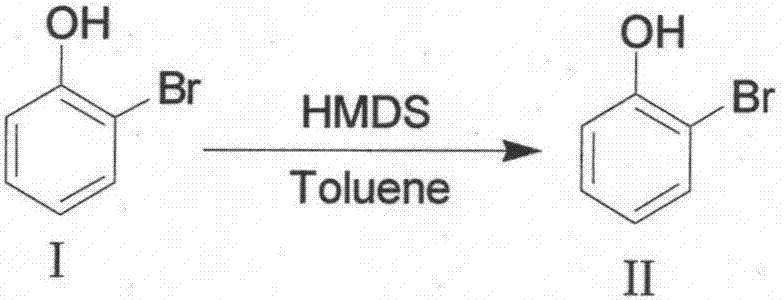
[0026] 1. Synthesis of compound II
[0027]
[0028] (1) Prepare a 10L glass reactor. After the Ar replacement is complete, add 4L of toluene and 2-bromophenol (1.2kg, 6.94mol) in sequence, then heat to reflux at an internal temperature of about 100-110°C;
[0029] (2) Under reflux conditions, slowly add hexamethyldisilazane (HMDS) (1.23kg, 7.63mol) dropwise, with heat generation and alkaline gas generation;
[0030] (3) After the dripping is finished, continue to reflux and mature for 1 to 2 hours at an internal temperature of 105 to 115°C;
[0031] (4) Sampling and testing. After GC confirms that the raw material 2-bromophenol has disappeared, the temperature of the water bath is cooled down, the reaction liquid is cooled to below 40°C, and conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com