Preparation method and application of PtPdCu electrocatalyst for fuel cells
An electrocatalyst, fuel cell technology, applied in the direction of fuel cells, battery electrodes, electrochemical generators, etc., can solve the problems of low reserves, restrict the commercialization process of fuel cells, high cost, and achieve large applications and development prospects, excellent electric power. Catalytic oxidation of methanol performance, easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Take 0.5 g triblock copolymer P123 and dissolve it in 20 mL double-distilled water by ultrasonic stirring; add 1 mL chloroplatinic acid hexahydrate solution (0.8 g / mL), 10 mg potassium chloropalladate , 3 mg of anhydrous copper chloride and 1 mg of potassium iodide, stirred, ultrasonically dissolved, and mixed uniformly. In the final mixed solution, the concentrations of chloroplatinic acid hexahydrate, potassium chloropalladate and copper chloride were all 0.03 mmol / L, Potassium iodide concentration is 0.01 mmol / L.
[0031] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, seal the reactor tightly, place it in a blast drying oven, and react at 180°C for 12 hours.
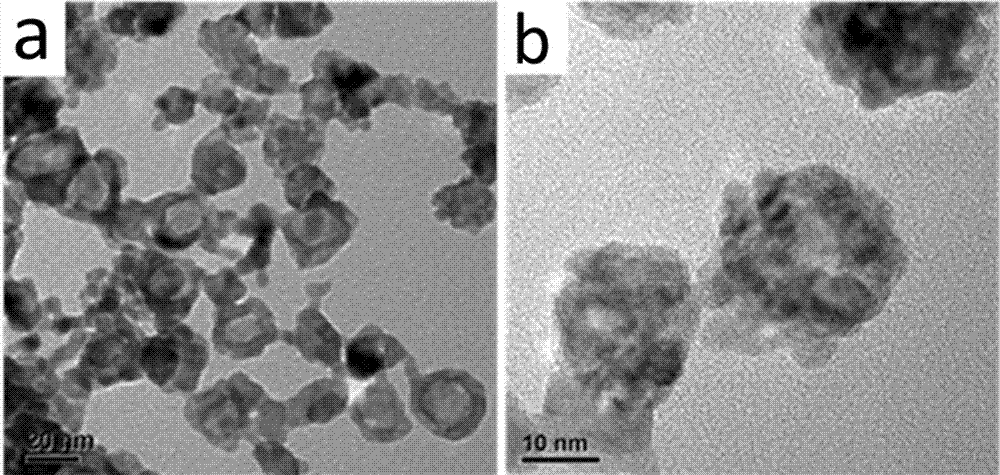
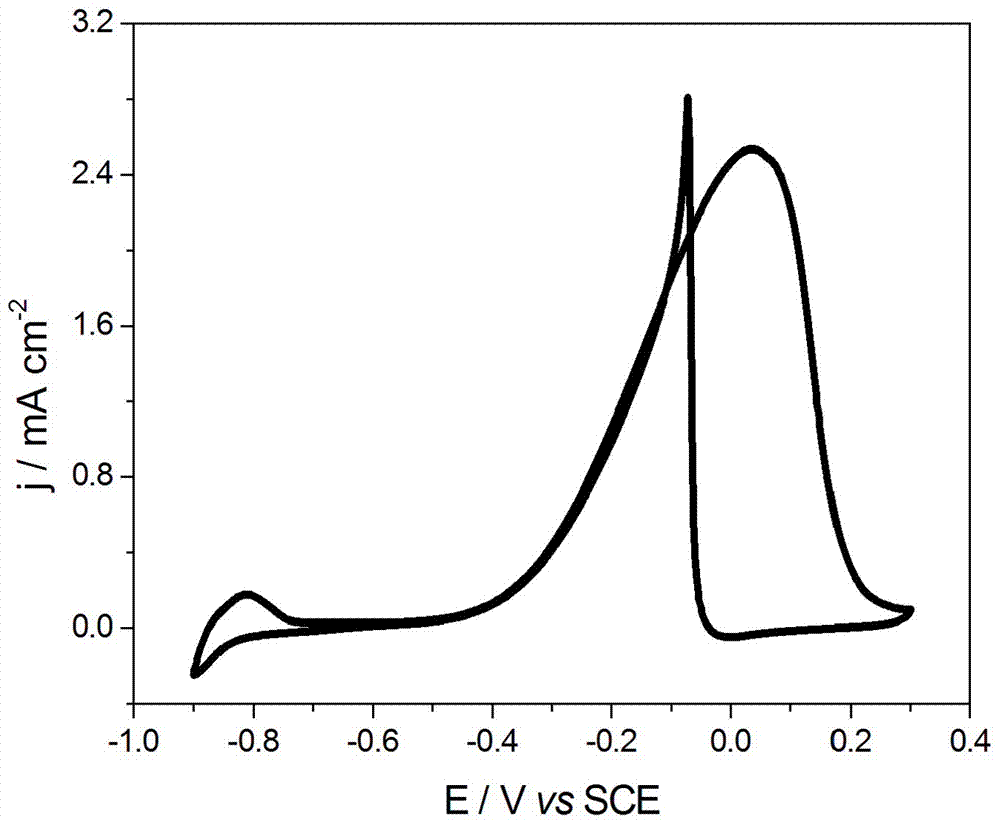
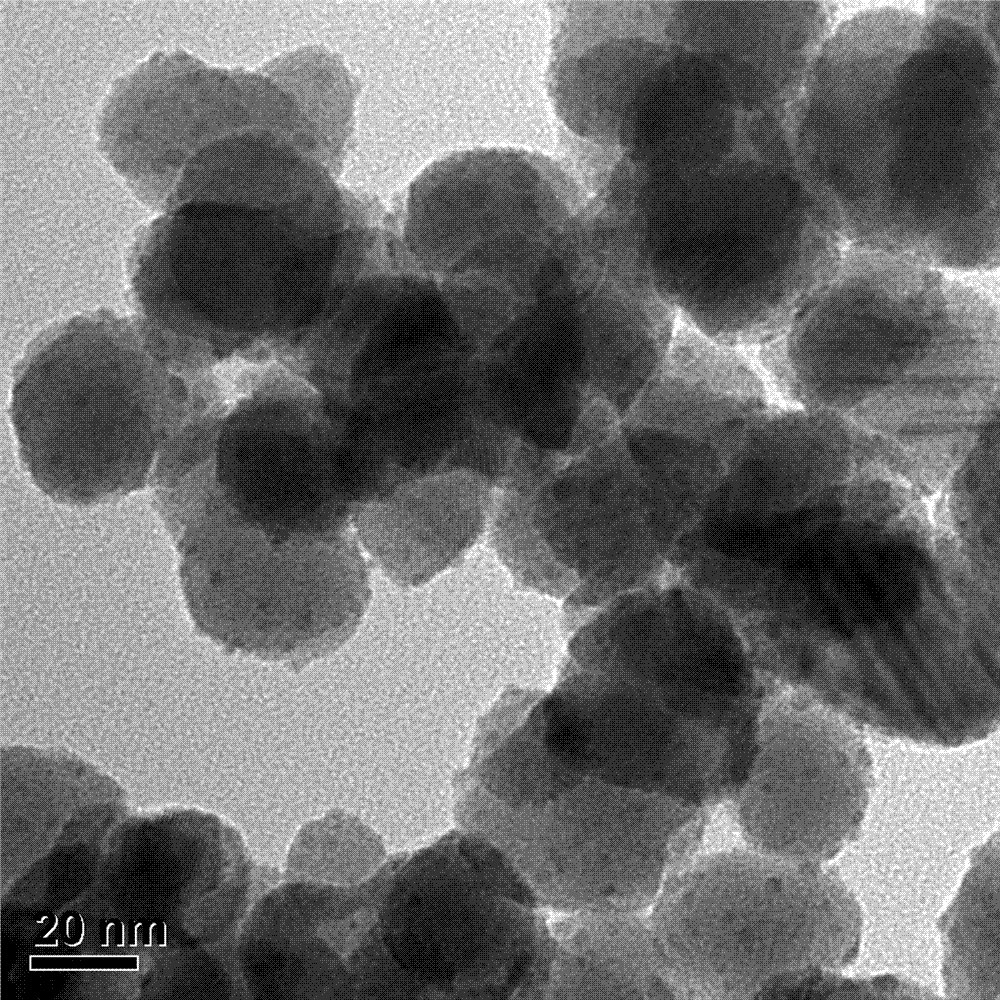
[0032] (3) Naturally cool to room temperature, centrifuge the black suspension obtained after the reaction at 10,000 r / min, wash twice with twice distilled water, then three times with absolute ethanol, and finally add the washed product to The PtPdCu electrocataly...
Embodiment 2
[0037] (1) Take 0.5 g triblock copolymer P123 and dissolve it in 20 mL double-distilled water by ultrasonic stirring; add 1 mL chloroplatinic acid hexahydrate solution (0.8 g / mL), 10 mg potassium chloropalladate , 3 mg of anhydrous copper chloride and 20 mg of potassium iodide, stirred, ultrasonically dissolved, and mixed uniformly. In the final mixed solution, the concentrations of chloroplatinic acid hexahydrate, potassium chloropalladate and copper chloride were all 0.03 mmol / L, Potassium iodide concentration is 0.03-0.05 mmol / L.
[0038] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, seal the reactor tightly, place it in a blast drying oven, and react at 180 °C for 12 hours.
[0039](3) Naturally cool to room temperature, centrifuge the black suspension obtained after the reaction at 10,000 r / min, wash twice with twice distilled water, then three times with absolute ethanol, and finally add the washed product to The PtPdCu electro...
Embodiment 3
[0044] (1) Take 0.5 g triblock copolymer P123 and dissolve it in 20 mL double-distilled water by ultrasonic stirring; add 1 mL chloroplatinic acid hexahydrate solution (0.8 g / mL), 10 mg potassium chloropalladate , 3 mg anhydrous copper chloride and 40 mg potassium iodide, stirred, ultrasonically dissolved, and mixed uniformly. In the final mixed solution, the concentrations of chloroplatinic acid hexahydrate, potassium chloropalladate and copper chloride were all 0.03 mmol / L, Potassium iodide concentration is 0.10 mmol / L.
[0045] (2) Transfer the mixed solution to the liner of a 50 mL polytetrafluoroethylene reactor, seal the reactor tightly, place it in a blast drying oven, and react at 180 °C for 12 hours.
[0046] (3) Naturally cool to room temperature, centrifuge the black suspension obtained after the reaction at 10,000 r / min, wash twice with twice distilled water, then three times with absolute ethanol, and finally add the washed product to The PtPdCu electrocatalyst f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com