Quantitative indoor quality control substance for whole-blood EBV-DNA
A technology of EBV-DNA and quantitative chamber, which is applied in the direction of measuring devices, preparation of test samples, instruments, etc., can solve the problems of lack of whole blood EBV quality control products, and achieve reliable test results, clear production process, and quality assurance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention.
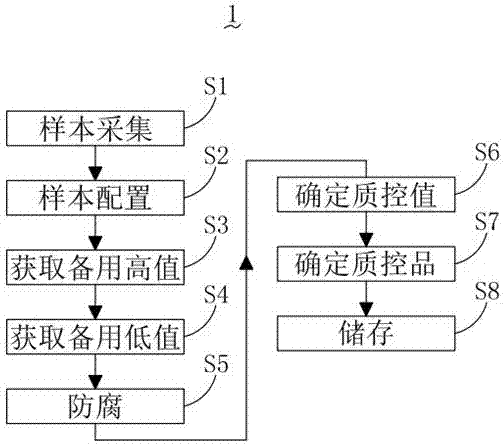
[0042] see figure 1 , is a flow chart of the preparation method 1 of the whole blood EBV-DNA quantitative internal quality control product provided by the present invention.
[0043] The preparation method 1 of the whole blood EBV-DNA quantitative indoor quality control product comprises the following steps:
[0044] S1: Sample collection:
[0045] Collect several whole blood samples with negative EBV-DNA test results, and the test results are greater than 10 6 Several whole blood samples of IU / mL are recorded as negative samples and positive samples respectively;
[0046] S2: Sample configuration:
[0047] Prepare three 45mL sample tubes, take 90mL of the negati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com