A kind of preparation method of atazanavir bisulfate crystal form h1
A bisulfate and atazanavir technology, which is applied in the field of preparation of atazanavir bisulfate crystal form H1, can solve the problems of low yield and high methanol content, and achieve high yield, high chromatographic purity, and repeatability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method used in Comparative Example 1 in the examples of the present invention is the preparation method of atazanavir crystal form H1 disclosed in patent WO2010079497.
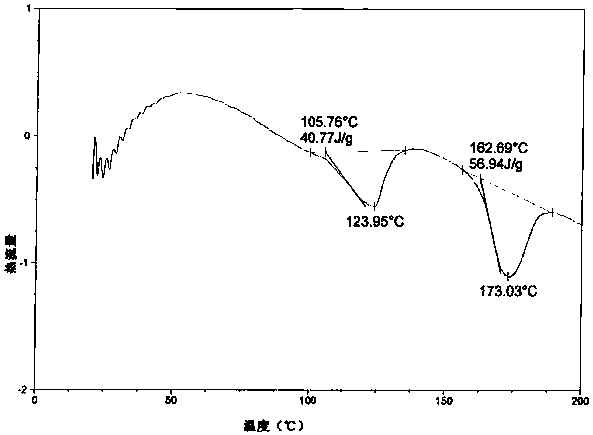
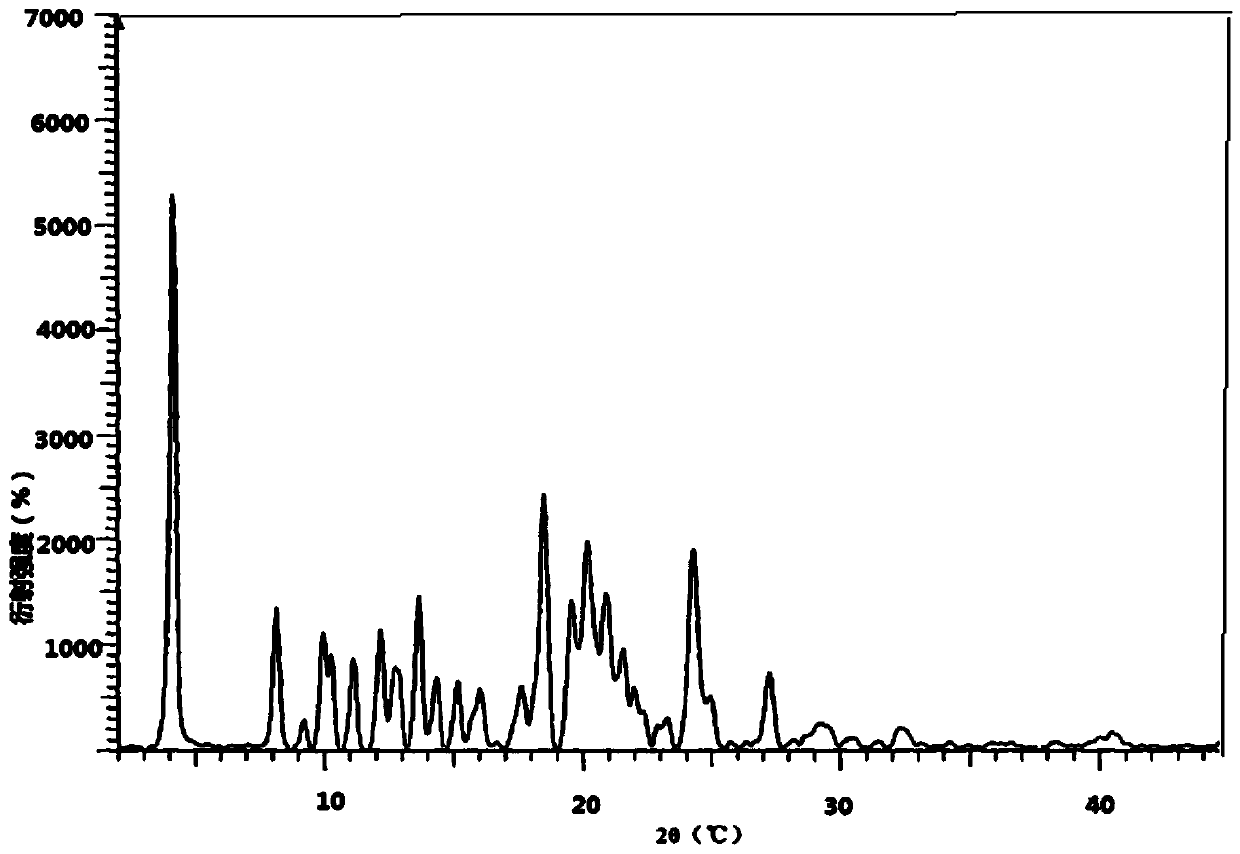
[0033] In the drawings of the present invention figure 2 and Figure 4 XRPD and DSC charts of atazanavir bisulfate crystalline form H1 disclosed in the cited patent WO2010079497.
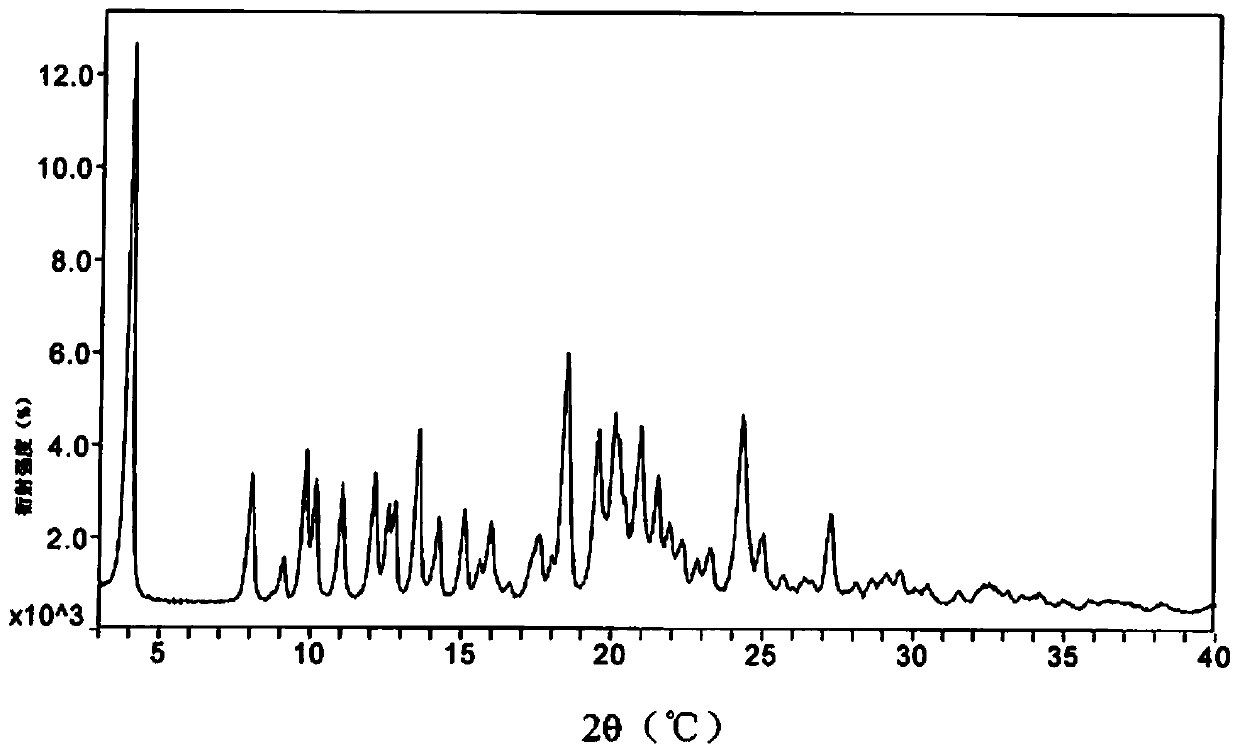
[0034] The powder X-diffraction testing instrument model involved in the present invention is: German Bruker D8 advance; testing conditions: Voltage, Current is 40Kv, 40mA, Stand-End Position is 0-40°2θ, Increment is 0.02°2θ, Time per step 0.5s, detection environment: 26°C, humidity 44%RH.
[0035] The model of the differential scanning calorimeter involved in the present invention is: TA Q200; the test method: Equlibrate at 20°C, Ramp at 10.0°C / min to 250.0°C, N 2 The flow rate is 40mL / min, the aluminum pan is covered. Testing environment: 25°C, humidity 55%RH.
[0036] Methanol detection method of the ...
Embodiment 1
[0037] Example 1: Preparation of Atazanavir Hydrogen Sulfate Form H1
[0038] A mixed solution of 30 g of methanol, 450 g of ethyl acetate and 30 g of butanone was added to 100 g of atazanavir hydrogen sulfate crystal form A, stirred at room temperature for 12 h, filtered, and the solid was washed with 30 g of butanone. The obtained product was vacuum-dried at 50-55°C for 8.0 hours to obtain an off-white solid. Yield: 96.5%, HPLC purity: 98.86%, methanol content: 0.13%. See XRPD figure 1 , see DSC image 3 .
Embodiment 2
[0039] Example 2: Preparation of Atazanavir Hydrogen Sulfate Form H1
[0040] A mixed solution of 50 g of methanol, 500 g of ethyl acetate and 50 g of butanone was added to 100 g of atazanavir hydrogen sulfate crystal form A, stirred at room temperature for 12 h, filtered, and the solid was washed with 3.0 g of butanone. The obtained product was vacuum-dried at 50-55°C for 8.0 hours to obtain an off-white solid. Yield: 95.3%, HPLC purity: 99.13%, methanol content: 0.15%. XRPD with figure 1 Consistent, DSC same image 3 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com