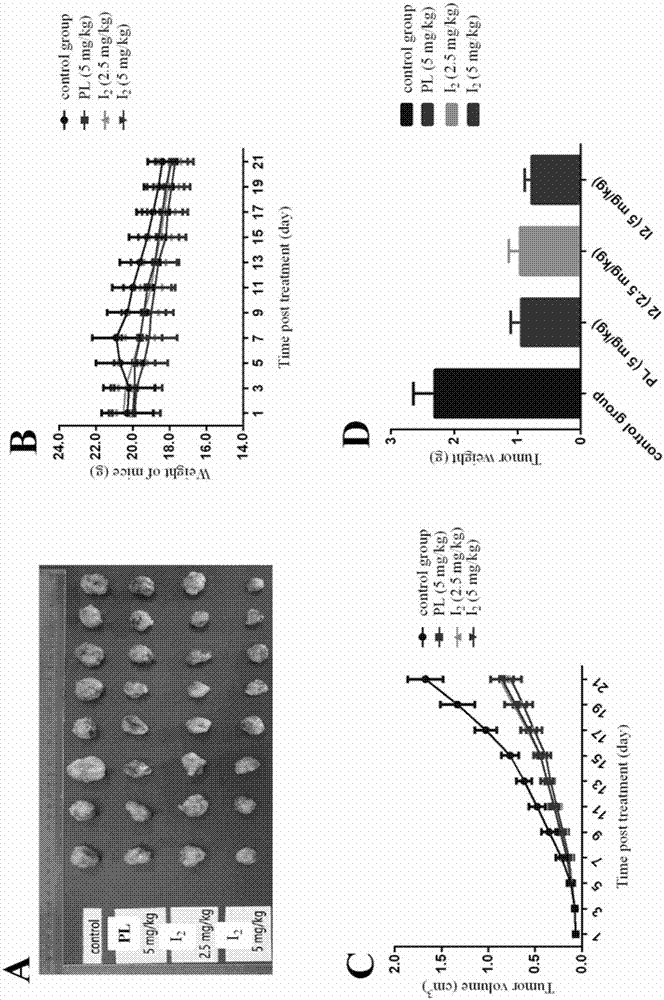
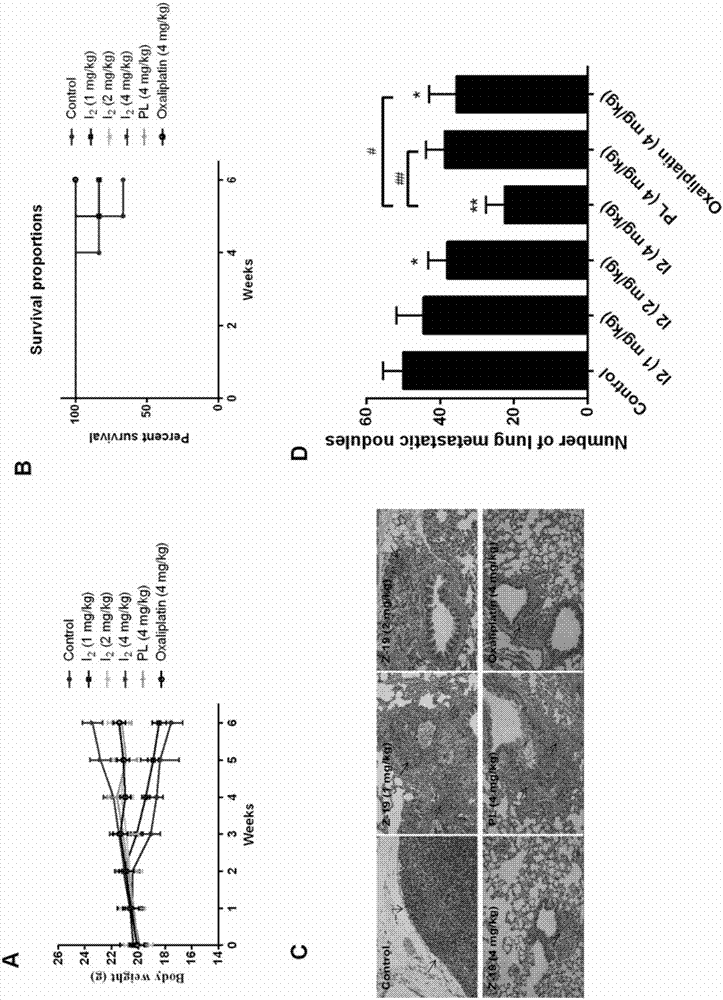
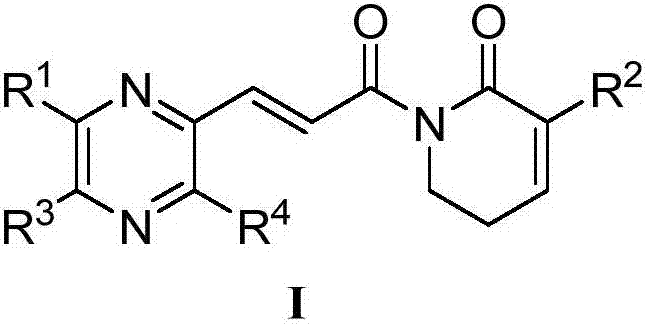
Piperlongumine-ligustrazine heterocomplex, preparation method and medical application
A technology of uses and compounds, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of changing pharmacokinetic properties, increasing toxic and side effects, and accumulating toxicity, achieving excellent anti-proliferative and metastatic activities of colon cancer cells, excellent effects, The effect of increasing ROS levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: (E)-1-{3-[2-(3,5,6-trimethylpyrazine)]acryloyl}-5,6-dihydropyridine-2(1H)-one (I 1 )
[0054] Intermediate V (0.5mmol) was dissolved in 20mL of anhydrous tetrahydrofuran, triethylamine (0.6mL) was added dropwise thereto, and pivaloyl chloride (0.6mL) was added dropwise to the reaction solution at -20°C, followed by reaction for 45 minutes At the same time, dissolve the intermediate VI (0.5mmol) in 20mL of anhydrous tetrahydrofuran, add n-butyllithium (2.9mL) at -70°C, and then stir for about 45 minutes, then directly add the reaction solution in the previous step to In this reaction, following reaction for 1 hour, the reaction was monitored by TLC. Add 10 mL of saturated ammonium chloride aqueous solution to the reaction liquid, quench the n-butyllithium in the reaction, extract, wash the aqueous phase with ethyl acetate (20 mL × 3), combine the organic phases and wash with saturated brine (30 mL × 3 ), dried over anhydrous sodium sulfate, concentrated to g...
Embodiment 2
[0056] Example 2: (E)-3-chloro-1-{3-[2-(3,5,6-trimethylpyrazine)]acryloyl}-5,6-dihydropyridine-2(1H) - Keto (I 2 )
[0057] Reference compound I 1 The synthetic method of obtaining pure product pale yellow solid (I 2 ) 236 mg. Yield 56%; mp 111-114°C. 1 H NMR (300MHz, CDCl 3 ), δ(ppm): 2.49(s, 6H, 2×CH 3 ),2.54~2.56(m,2H,NCH 2 CH 2 ),2.59(s,3H,CH 3 ),4.06(t,J=6.0Hz,2H,N CH 2 CH 2 ), 7.06(t, J=4.5Hz, 1H, COClC= CH ),7.82~7.95(m,2H,CH=CH); 13 C NMR (75MHz, CDCl 3 ):δ21.0,21.9,22.2,25.5,42.0,126.2,128.3,138.4,141.3,143.2,149.3,150.1,152.6,161.4,168.8; ESI-MS:328.1[M+Na] + ; HRMS calculated for C 15 h 16 N 3 o 2 ClNa[M+Na] + 328.0829, found 328.0835, ppm error 1.8.
Embodiment 3
[0058] Example 3: (E)-3-bromo-1-{3-[2-(3,5,6-trimethylpyrazine)]acryloyl}-5,6-dihydropyridine-2(1H) - Keto (I 3 )
[0059] Reference compound I 1 The synthetic method of obtaining pure product pale yellow solid (I 3 ). Yield 43%; mp 109-113°C. 1 HNMR (300MHz, CDCl 3 ), δ(ppm):2.44~2.59(m,11H,NCH 2 CH 2 and 3×CH 3 ), 4.06(t, J=7.5Hz, 2H, N CH 2 CH 2 ), 7.06(t, J=4.5Hz, 1H, COClC= CH ),7.83~7.93(m,2H,CH=CH); 13 C NMR (75MHz, CDCl 3 ):δ21.0,21.9,22.2,26.9,42.1,113.2,126.3,138.3,143.3,147.3,149.3,150.1,152.6,161.3,169.0; ESI-MS:372.0[M+Na] + ; HRMS calculated for C 15 h 16 N 3 o 2 BrNa[M+Na] + 372.0368, found 372.0371, ppm error 0.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com