A method for separating 25r- and 25s- epimers of ergostane-type triterpenes
An epimer, ergostane-type technology is applied in the field of splitting ergostane-type triterpenes 25R- and 25S-epimer, and can solve the problem of poor solubility and limitation of ergostane-type triterpenes. High efficiency and cost of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
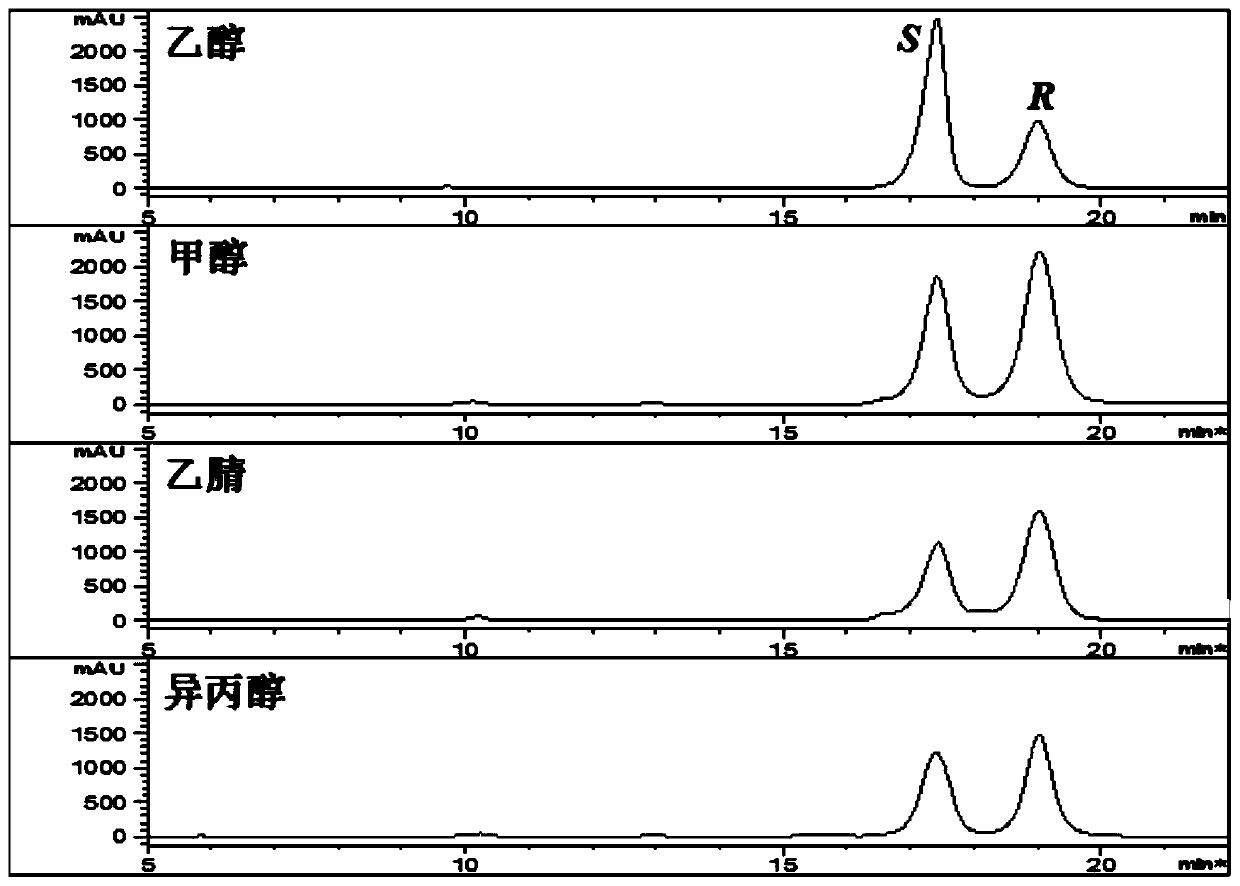
[0029] Example 1. Effects of different organic reagents on the separation of 25R / S-antcin K epimers.
[0030] 1. Experimental materials and methods.
[0031] All chemical reagents mentioned in this method were purchased from Beijing Chemical Plant.
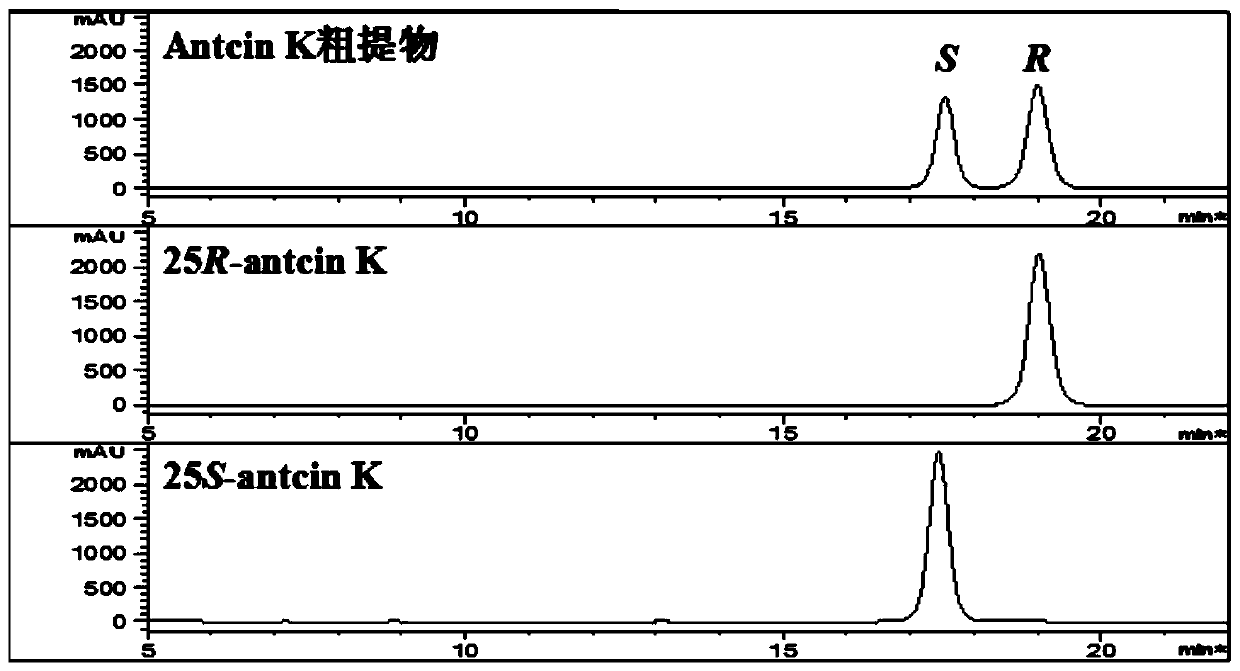
[0032] 25R-antcin K and 25S-antcin K standard curves: Accurately weigh 25R-antcin K and 25S-antcin K reference substances, dissolve in methanol, make 5.00mg / mL mother solution, and dilute step by step with methanol to 2.5, 1, 0.5 ,0.25,0.1,0.05mg / mL concentration, 0.22μm microporous membrane filtration, 10μL injection detection.
[0033] Detection of antcin K crude extract: Weigh 1.00 mg of antcin K crude extract powder, dissolve in 1000 μL methanol, filter through a 0.22 μm microporous membrane, and inject 10 μL for detection.
[0034]Chromatographic conditions:
[0035] Instrument: Agilent 1100 high performance liquid chromatography;
[0036] Chromatographic column: YMC-Pack ODS-A column (5μm, 4.6×250mm);
[0037] Elution p...
Embodiment 2
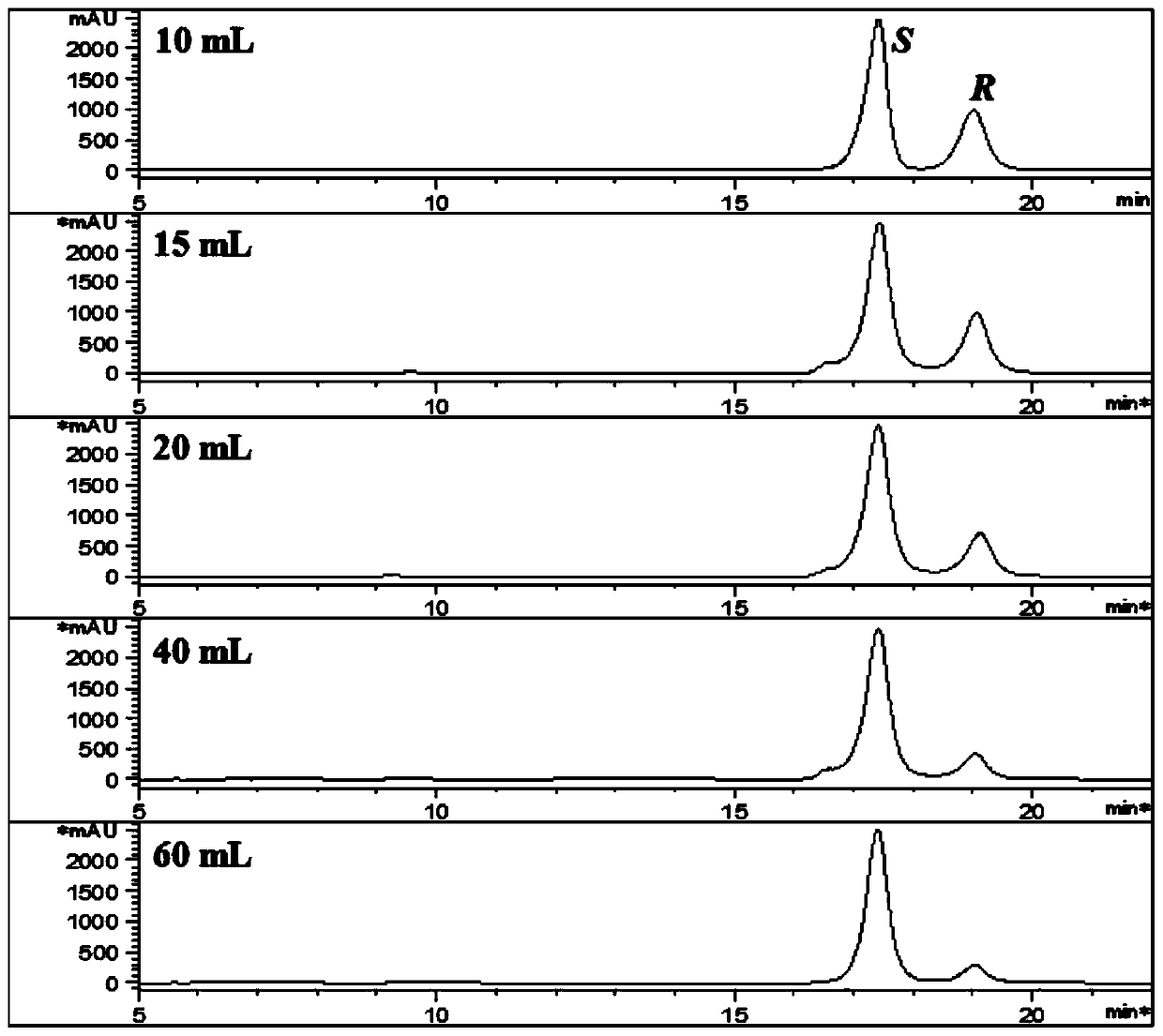
[0049] Example 2. Effects of different volumes of ethanol on the separation of 25R / S-antcin K epimers.
[0050] 1. Experimental materials and methods.
[0051] Weigh 1g of antcin K crude extract solid powder, add 10, 15, 20, 40, 60mL of ethanol respectively, stir evenly, let stand for 15min, and vacuum filter to dryness. The filter residue was dissolved in methanol and injected for analysis.
[0052] 2. Experimental results
[0053] The experimental results are shown in Table 2 and image 3 . As the volume of ethanol increases, the relative content of 25S-antcin K also increases, but the quality of filter residue decreases and the yield decreases. 15 times the volume of ethanol has a good effect on the separation of antcin K.
[0054] Table 2
[0055]
[0056]
Embodiment 3
[0057] Example 3. Effects of different dissolution times on the separation of 25R / S-antcin K epimers.
[0058] 1. Experimental materials and methods.
[0059] Weigh 10.0 mg of antcin K pure solid powder (relative purity >90%), add 150 μL of ethanol, vortex evenly, let stand, and wait for 20s, 30s, 1min, 2min, 5min, 15min, 30min, 1h, 2h, 4h , 8h, 12h, 24h, 36h, 48h, 72h membrane filtration. The filtrate was diluted 50 times with methanol and injected for analysis.
[0060] 2. Experimental results
[0061] The experimental results are shown in Table 3 and Figure 4 . The relative content of 25R-antcin K in ethanol solution gradually increased with time, but the total dissolved mass of 25R / S-antcin K remained basically unchanged. The relative content of 25R-antcin K in the solution can reach a higher level after 15 minutes of dissolution.
[0062] table 3
[0063]
[0064]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com