C20H18N6Na2O7S4 drug entity composition and preparation for children
A technology of c20h18n6na2o7s4 and composition, applied in the pharmaceutical field, can solve the problems of leakage of water-soluble drugs, low encapsulation efficiency, difficulty in achieving encapsulation efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
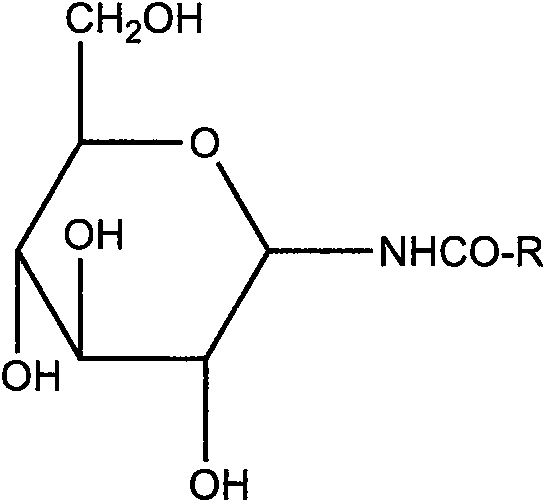
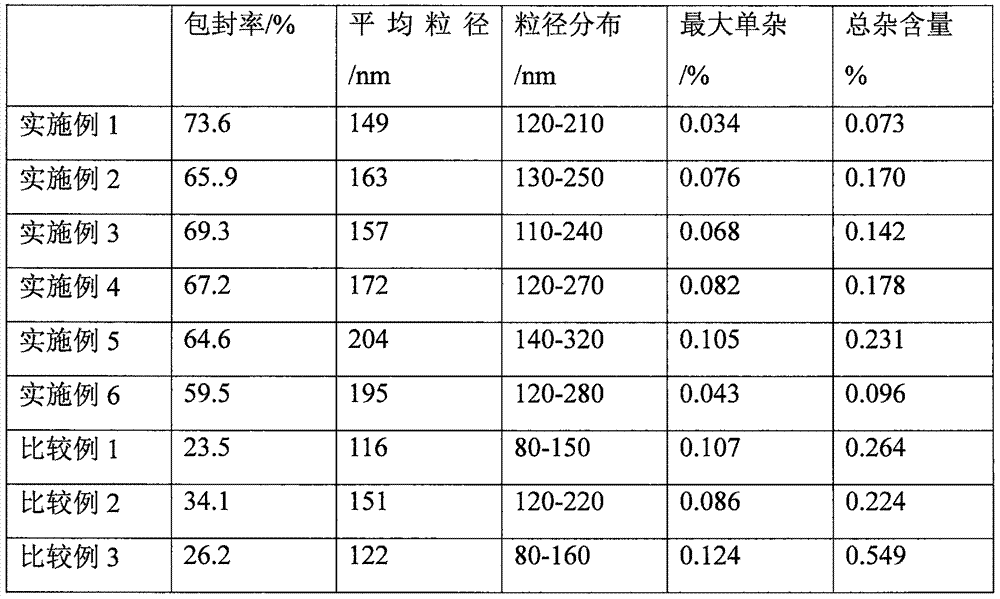
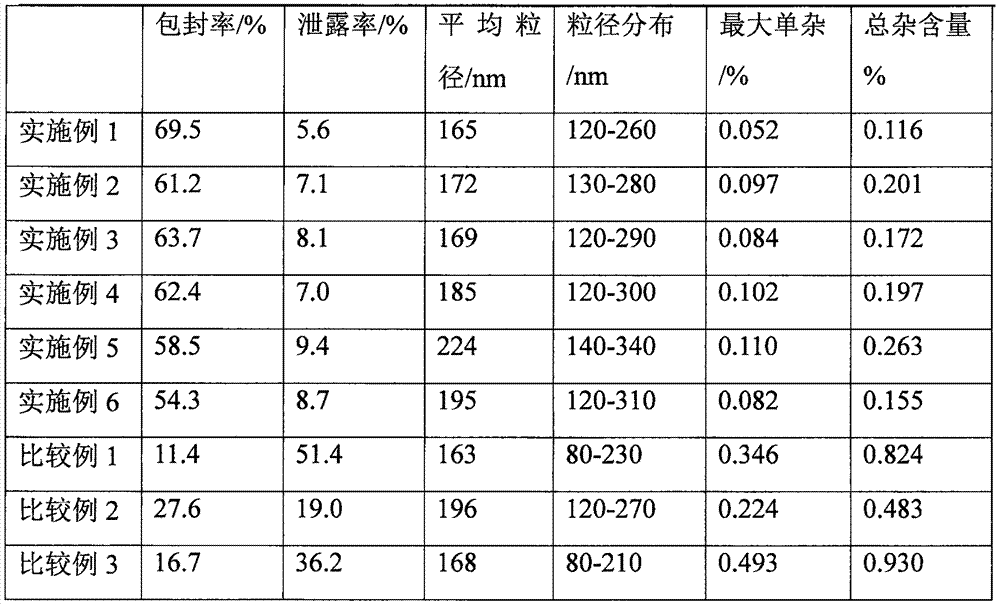
[0047] Weigh 3.6 g of hydrogenated soybean lecithin and 1.0 g of cholesterol as a film-forming substance, and 0.14 g of N-(β-D-glucopyranose) octanamide as a film-forming substance stabilizer, and dissolve the above mixture with 120 mL of ether; To which was added containing 0.135gC 20 h 18 N 6 Na 2 o 7 S 4 20mL of PBS buffer solution with a pH value of 6.0, ultrasonically make the mixed system into a homogeneous system; evaporate the organic solvent under reduced pressure until a gel is formed, add 10mL of PBS buffer solution with a pH value of 6.0 for hydration reaction, and the temperature is 30°C , the hydration time is 2h; then continue to evaporate under reduced pressure for 15 minutes; after ultrasonication, let it stand for 3h at a temperature of 4°C to obtain the above C 20 h 18 N 6 Na 2 o 7 S 4 Composition of pharmaceutical entities.
Embodiment 2
[0049] Weigh 3.6 g of hydrogenated soybean lecithin and 1.0 g of cholesterol as a film-forming substance, and 0.131 g of N-(β-D-glucopyranose) octanamide as a film-forming substance stabilizer, and dissolve the above mixture with 120 mL of ether; To which was added containing 0.135gC 20 h 18 N 6 Na 2 o 7 S 4 15mL of PBS buffer solution with a pH value of 5.5, ultrasonically make the mixed system into a homogeneous system; evaporate the organic solvent under reduced pressure until a gel is formed, add 5mL of PBS buffer solution with a pH value of 5.5 for hydration reaction, and the temperature is 35°C , the hydration time is 2h; then continue to evaporate under reduced pressure for 15 minutes; after ultrasonication, let it stand for 1h at a temperature of 4°C to obtain the above C 20 h 18 N 6 Na 2 o 7 S 4 Composition of pharmaceutical entities.
Embodiment 3
[0051] Weigh 3.6 g of hydrogenated soybean lecithin and 1.0 g of cholesterol as a film-forming substance, and 0.175 g of N-(β-D-glucopyranose) octanamide as a film-forming substance stabilizer, and dissolve the above mixture with 120 mL of ether; To which was added containing 0.135gC 20 h 18 N 6 Na 2 o 7 S 4 30mL of PBS buffer solution with a pH value of 6.5, ultrasonically make the mixed system into a homogeneous system; evaporate the organic solvent under reduced pressure until a gel is formed, add 10mL of PBS buffer solution with a pH value of 6.5 for hydration reaction, and the temperature is 25°C , the hydration time is 2h; then continue to evaporate under reduced pressure for 15 minutes; after ultrasonication, let it stand for 4h at a temperature of 4°C to obtain the above C 20 h 18 N 6 Na 2 o 7 S 4 Composition of pharmaceutical entities.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com