Perovskite-like material CsPb2Br5, as well as synthetic method and application thereof
A technology of perovskite material and synthesis method, applied in the field of nanomaterials, can solve the problems of harsh conditions, complex process, unfavorable recycling of organic waste liquid, etc., achieve cost saving, avoid toxic and harmful solvents, and be suitable for large-scale mass production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A kind of perovskite material CsPb 2 Br 5 The synthetic method specifically comprises the following steps;
[0029] Step 1, dissolving the cesium source in a solvent, stirring and dissolving to form a clear solution;
[0030] Step two, the PbBr 2 Mix with a clear solution and react at room temperature, cesium, lead, and bromide ions undergo ion exchange to form CsPb 2 Br 5 Perovskite-like precipitation.
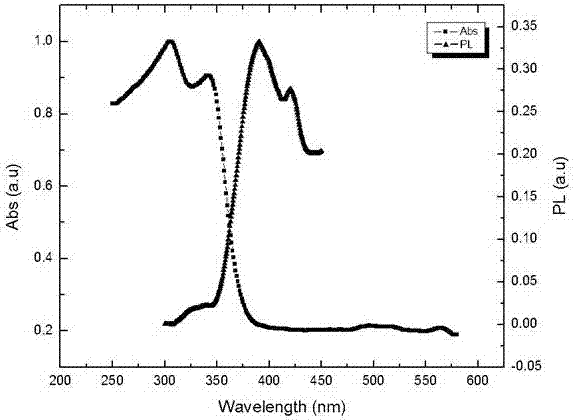
[0031] The method utilizes hydrolysis and chemical reaction to separate and ion-exchange cesium, lead, and bromide ions, because the product CsPb 2 Br 5 Insoluble in solvents and precipitated out. The obtained perovskite-like material CsPb 2 Br 5 Spectral emission in the ultraviolet region can be achieved. The used solvent is green and environmentally friendly, harmless to human body and environment, saves cost, the reaction temperature can be carried out at room temperature, the operation is simple and easy, and it is suitable for large-scale batch productio...
Embodiment 1
[0045] 1. Add 1mmol of CsAc into the reaction flask, then use 2ml of deionized water as solvent, stir to dissolve to form a clear solution, then add 2.5mmol of PbBr 2 Add to reaction flask.
[0046] 2. After a period of reaction, cesium, lead, and bromide ions are separated and ion exchanged, and the resulting product is precipitated by chemical co-precipitation.
[0047] 3. The formed precipitate is transferred and purified by washing with HBr to obtain the perovskite-like material CsPb 2 Br 5 .
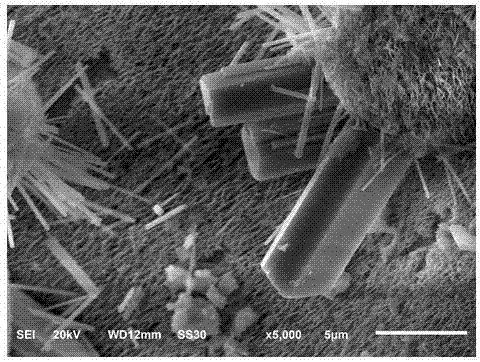
[0048] 4. The above-mentioned perovskite-like material CsPb 2 Br 5 Product morphology SEM picture as figure 2 shown.
Embodiment 2
[0050] 1. Add 1mmol of CsAc into the reaction flask, then use 2ml of deionized water and absolute ethanol as solvents, the volume ratio of ionized water and absolute ethanol is 1:3, stir to dissolve to form a clear solution.
[0051] 2. Add 2.5mmol of PbBr 2 Add it into the reaction bottle, and after a period of reaction, the cesium, lead, and bromide ions are separated and ion exchanged, and the resulting product is precipitated by chemical co-precipitation.
[0052] 3. The formed precipitate is transferred and purified by washing with HBr to obtain the perovskite-like material CsPb 2 Br 5 .
[0053] 4. The above-mentioned perovskite-like material CsPb 2 Br 5 Product morphology SEM picture as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com