Adjuvant-free single-dose HBsAg nanogel vaccine and preparation method thereof
A nanogel, single-dose technology, applied in the field of biomedicine, can solve the problems of high frequency of aluminum adjuvant vaccine immunization, short immunization memory time of aluminum adjuvant vaccine, and affecting the safety of the vaccinated population, achieving good immune effect, The effect of avoiding adverse reactions and reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
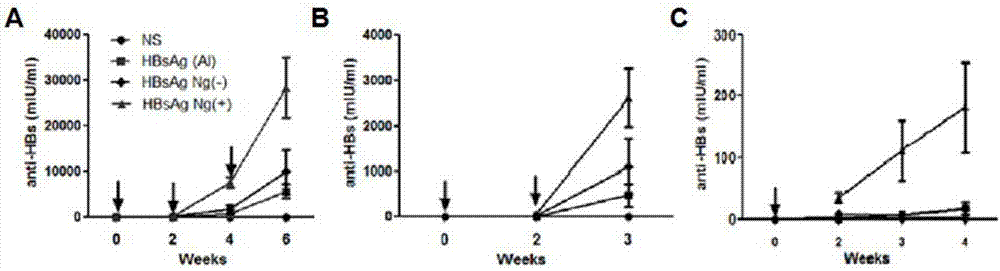
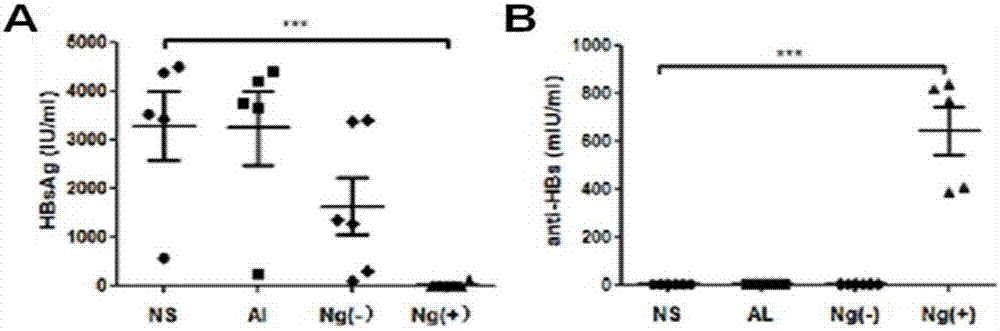
[0045] Example 1: Preparation of a single-dose HBsAg nanogel vaccine without an adjuvant with a positive charge on the surface
[0046] The specific preparation method is as follows:
[0047] CS solution (2mg / ml, pH=6), was added dropwise into HBsAg solution (25μg / ml) under stirring, after the stirring was stable, was added dropwise into γ-PGA solution (0.5mg / ml), stirred at 40°C for 20 minutes, that is A single-dose HBsAg nanogel vaccine (HBsAg Ng(+)) without adjuvant was prepared.
Embodiment 2
[0048] Example 2: Preparation of adjuvant-free single-dose HBsAg nanogel vaccine with negative charges on the surface
[0049] The specific preparation method is as follows:
[0050] γ-PGA solution (2mg / ml), was added dropwise to HBsAg solution (25μg / ml) under stirring, and after the stirring was stable, was added dropwise to CS solution (0.5mg / ml, pH=6), stirred at 40°C for 20 minutes, that is A single-dose HBsAg nanogel vaccine (HBsAg Ng(-)) without adjuvant was prepared.
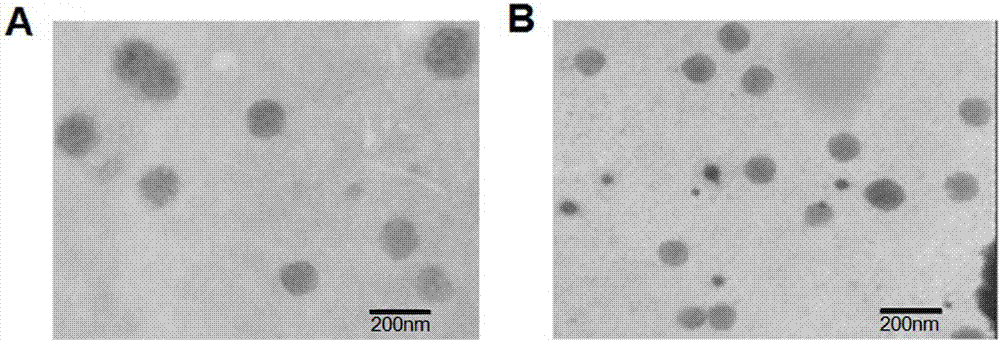
[0051] The physical and chemical properties of the non-adjuvanted single-dose HBsAg nanogel vaccine prepared in Example 1 and Example 2 were investigated:
[0052] The particle size and Zeta potential were measured by a nanoparticle size analyzer (Zetasizer NanoZS90) produced by Malvern Instruments, UK. Morphology by JEM-100CXII transmission electron microscope (Jeol Corporation, Japan). The encapsulation efficiency of HBsAg was indirectly measured by centrifuging (40000×g, 20min) and measuring the fre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com