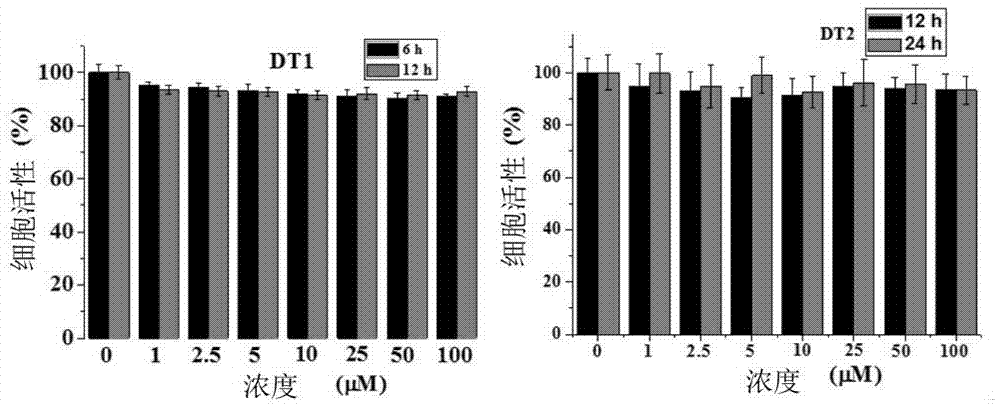
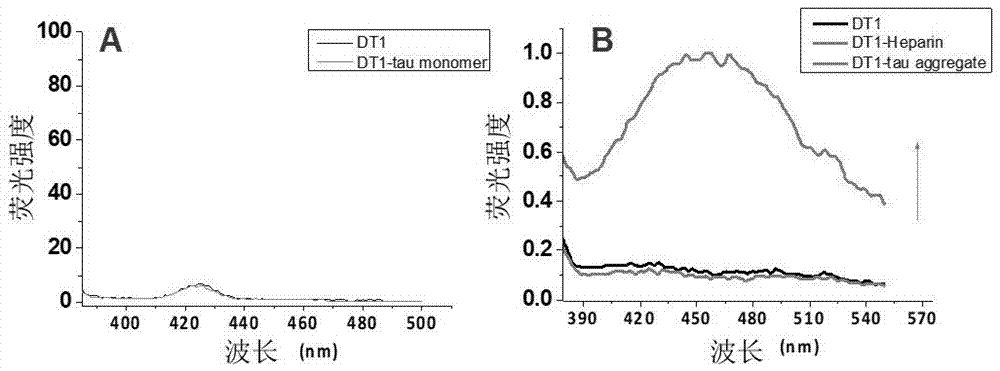
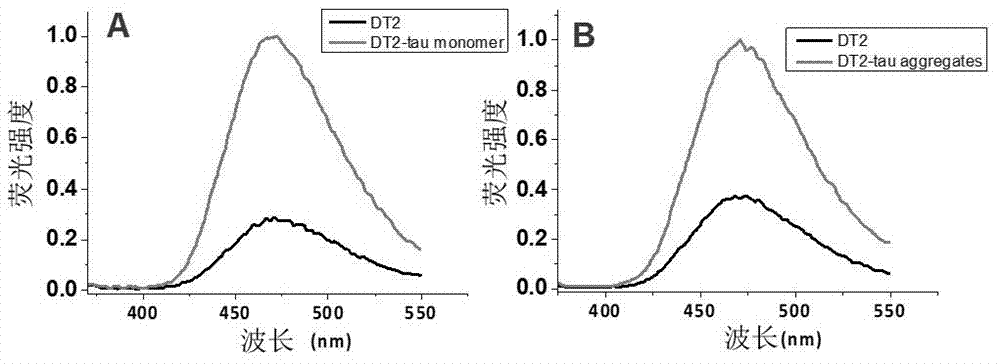
Molecule specifically bind to Tau protein and capable of inhibiting Tau protein aggregation, preparation method and application thereof
A technology for protein aggregation and specificity, applied in chemical instruments and methods, analytical materials, drug combinations, etc., can solve problems such as low specificity and inability to inhibit aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (n=1):
[0031] Preparation of compound 5: 1-bromo-2-(2-bromomethoxy)ethane (37.0 μL, 0.3 mmol), 2-chloroquinolin-6-ol (116 mg, 0.65 mmol) and potassium carbonate (96.7 mg, 0.7 mmol) was dissolved in DMF (2.5 mL), kept at 70° C. for 4 h, followed by TLC until the reaction was complete. After the reaction, the reaction liquid was extracted with toluene-water system, the filtrate was dried with magnesium sulfate, concentrated under reduced pressure, separated and purified by column chromatography to obtain compound 10 (red solid, 95.8%; ethyl acetate:petroleum ether=1:2 ).
[0032] 1 H NMR (400MHz, CDCl 3 ): δ=7.96-7.90 (m, 4H); 7.41 (dd, J 1 =2.4Hz,J 2 =9.2Hz, 2H); 7.32(d, J=8.4Hz, 2H); 7.07(d, J=2.4Hz, 2H); 4.29(t, J=4.4Hz, 4H); 4.04-4.02(m, 4H ).
[0033] 13 C NMR: (125MHz, CDCl 3 ), δ=157.2, 148.2, 143.8, 137.6, 130.0, 127.8, 123.2, 122.6, 106.3, 69.9, 67.9.
[0034] HR-MS(ESI,m / z):calcd for C 22 h 9 N 2 o 3 Cl 2 [M+H] + ,429.0773,found 42...
Embodiment 2
[0039] Embodiment 2 (n=1.5):
[0040] Preparation of compound 6: 2-chloroquinolin-6-alcohol ((253mg, 1.1mmol) was dissolved in dry THF, and then PPh was added successively thereto 3(393mg, 1.5mmol), triethylene glycol (68.3μL, 0.5mmol) and DIAD (diisopropylazodicarboxylate, 294μL, 1.5mmol). The reaction was kept at room temperature for 4 h, followed by TLC until the reaction was complete. After the reaction, concentrated under reduced pressure, separated and purified by column chromatography to obtain compound 6 (red solid, 95.8%; ethyl acetate:dichloromethane=1:1). 1 H NMR (400MHz, CDCl 3 ):δ=7.94-7,88(m,4H); 7.39(dd,J 1 =2.8Hz,J 2 =9.2Hz, 2H); 7.30(d, J=8.8Hz, 2H); 7.03(s, d, J=2.8Hz, 2H); 4.21(t, J=4.8Hz, 4H); 3.94-3.92(s ,4H); 3.79(s,4H).
[0041] Preparation of compound without DT2: Add compound 6 (255 mg, 0,54 mmol), Pd(PPh 3 ) 4 (116mg, 0.1mmol), 4-dimethylaminophenylboronic acid (162.1mg, 1.62mmol), K 2 CO 3 (138.2g, 1.7mmol), under nitrogen atmosphere, add s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com