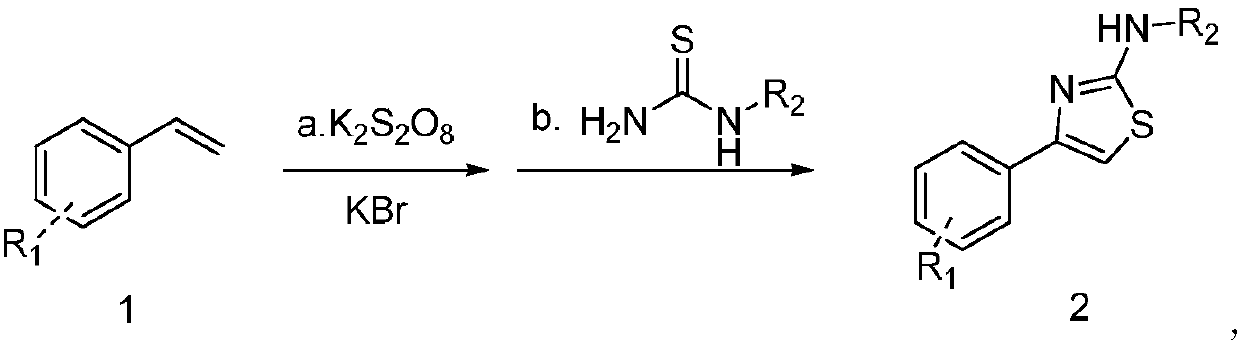
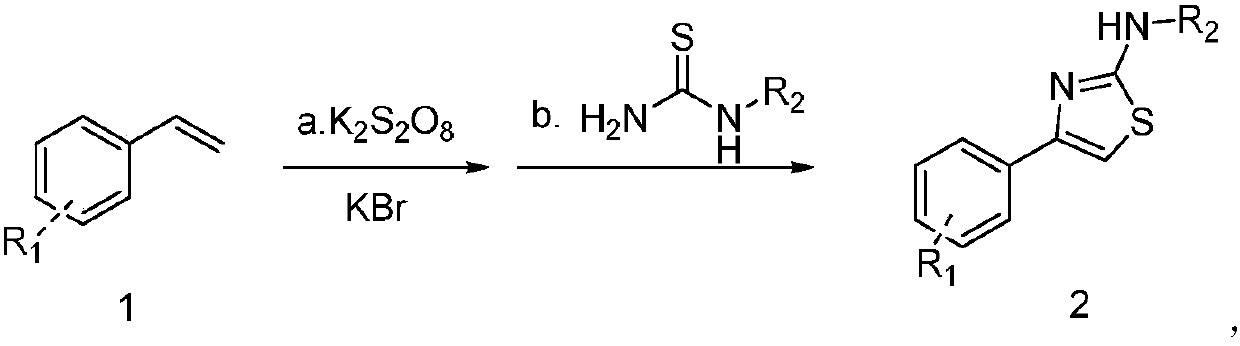
Synthesis method of novel thiazole ring derivative
A synthesis method and technology of thiazole rings, which are applied in the field of synthesis of new thiazole ring derivatives, can solve the problems of difficult control, severe reaction conditions, complicated operation, etc., and achieve high reaction yield and reduction of acidic and basic substances and metals The effect of using and reducing the discharge of three wastes and the pollution of the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of 2-amino-4-phenylthiazole
[0019] Add 3mL water into a 25mL reaction flask, then add 0.5mmol styrene, 1.25mmol K 2 S 2 o 8 and 1.25mmol KBr, reacted at 60°C for 12h, then added 0.5mmol thiourea, and continued to react at 60°C for 1h. After the reaction was completed, ethyl acetate was added for extraction, the organic phase was concentrated, and column chromatography gave 75mg of a white solid. The yield was 85%.
[0020] Product characterization: 1 H NMR (CDCl 3 ,600MHz)δ:7.81-7.75(m,2H),7.38(td,J=7.9,2.2Hz,2H),7.32-7.27(m,1H),6.72(d,J=2.4Hz,1H),5.29 (s,2H).
Embodiment 2
[0022] Synthesis of 2-amino-4-(p-bromophenyl)thiazole
[0023] Add 3mL water into a 25mL reaction flask, then add 0.5mmol p-bromostyrene, 1.25mmol K 2 S 2 o 8 and 1.25mmol KBr, reacted at 60°C for 12h, then added 0.5mmol thiourea, continued to react at 60°C for 1h, after the reaction was completed, added ethyl acetate for extraction, concentrated the organic phase, and obtained 105mg of white solid by column chromatography. The yield was 83%.
[0024] Product characterization: 1 H NMR (CDCl 3 ,600MHz):7.65(dt,J=8.4,1.6Hz,1H),7.50(dt,J=8.5,1.6Hz,1H),6.74(d,J=1.8Hz,1H),4.96(s,1H) .
Embodiment 3
[0026] Synthesis of 2-amino-4-(m-bromophenyl)thiazole
[0027] Add 3mL water to a 25mL reaction flask, then add 0.5mmol m-bromostyrene, 1.25mmol K 2 S 2 o 8 and 1.25mmol KBr, reacted at 60°C for 12h, then added 0.5mmol thiourea, continued to react at 60°C for 1h, after the reaction was completed, added ethyl acetate for extraction, concentrated the organic phase, and obtained 206mg of white solid by column chromatography. The yield was 81%.
[0028] Product characterization: 1H NMR (CDCl 3 ,600MHz):7.94(s,1H),7.68(d,J=7.8Hz,1H),7.41(d,J=7.9Hz,1H),7.23(t,J=8.0Hz,1H),6.74(s ,1H), 5.16(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com