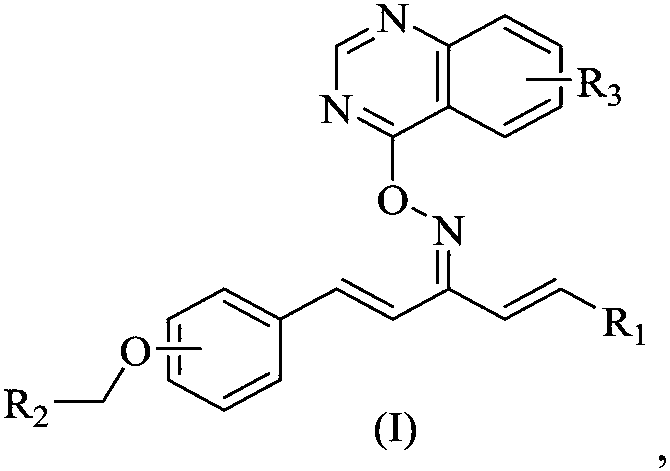
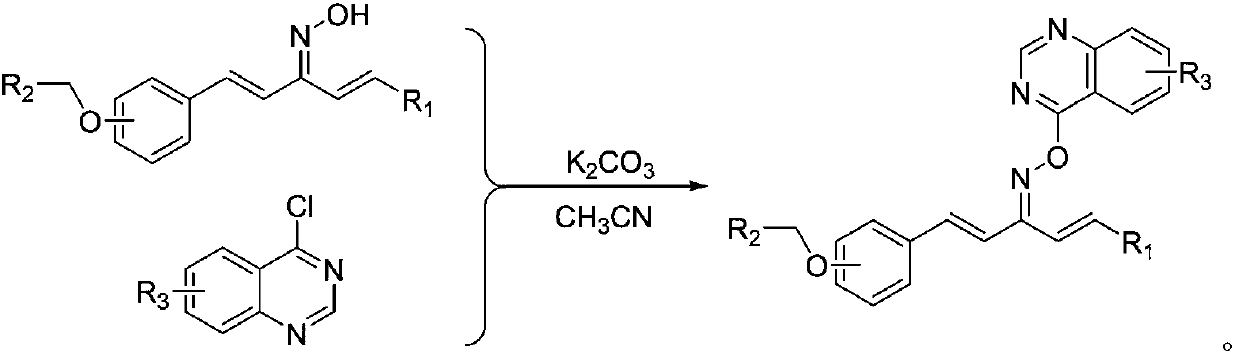
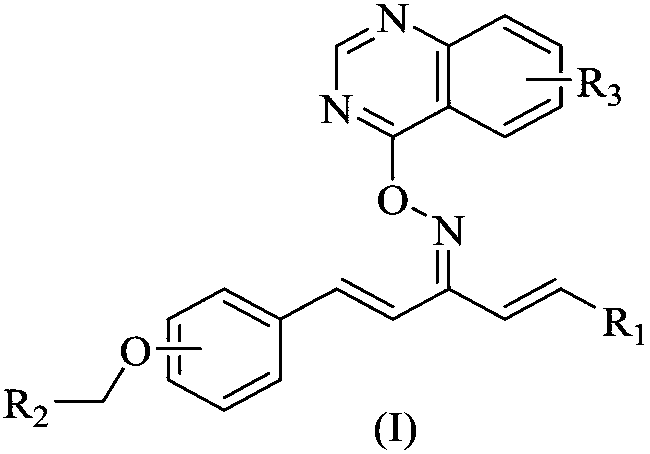
Quinazoline-containing 1,4-pentadiene-3-ketone oxime ether derivative as well as preparation method and application thereof
A technology of pentadiene and quinazoline, which is applied in the preparation of pentadiene ketone oxime ether derivatives and the application field of anti-plant virus, which can solve the problems that have not been seen before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (4-quinazolinyl)-1-(4-(3-methylbenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadiene-3-ketoxime ether Synthesis (compound number is I 1 ), including the following steps:
[0048] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one:
[0049]Add 4-hydroxybenzaldehyde (50mmol) into 60mL of acetone, stir for about 15min, ice-bath the reaction system for about 30min, add about 100mL of 5% NaOH solution to the system, and remove the In an ice bath, stir at room temperature for about 24 hours. After the reaction is over, transfer the system to a 500mL beaker and add an appropriate amount of ice water, and then use 5% dilute hydrochloric acid solution to adjust the pH of the system to about 5-6. After a large amount of yellow solids precipitate, extract the solids, and finally use ethanol to / water system recrystallized to obtain a yellow solid with a yield of 68%.
[0050] (2) Synthesis of 1-(2-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one:
[0051] Add 4-(hydroxyphenyl)-3-buten...
Embodiment 2
[0061] Synthesis of (4-chloroquinazolinyl)-1-(4-(2-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadiene-3-ketoxime ether (the compound number is I 2 ), including the following steps:
[0062] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one:
[0063] As in the first (1) step of Example 1.
[0064] (2) Synthesis of 1-(2-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one:
[0065] As in embodiment 1 (2) step.
[0066] (3) Synthesis of 1-(4-(2-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one:
[0067] As in step (3) of Example 1, the difference is that 1-(2-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one and o-chlorobenzyl chloride are used as raw materials .
[0068] (4) Synthesis of 1-(4-(2-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one oxime:
[0069] As in step (4) of Example 1, the difference is that 1-(4-(2-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one For the raw material.
[0070] (5) Synthesis of 4-chloroquinazoline:
[0071] As i...
Embodiment 3
[0075] Synthesis of (4-chloroquinazolinyl)-1-(4-(4-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadiene-3-ketoxime ether (the compound number is I 3 ), including the following steps:
[0076] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one:
[0077] As in the first (1) step of Example 1.
[0078] (2) Synthesis of 1-(2-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one:
[0079] As in embodiment 1 (2) step.
[0080] (3) Synthesis of 1-(4-(4-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one:
[0081] As in step (3) of Example 1, the difference is that 1-(2-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one and p-chlorobenzyl chloride are used as raw materials .
[0082] (4) Synthesis of 1-(4-(4-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one oxime:
[0083] As in step (4) of Example 1, the difference is that 1-(4-(4-chlorobenzyloxy)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one For the raw material.
[0084] (5) Synthesis of 4-chloroquinazoline:
[0085] As i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com