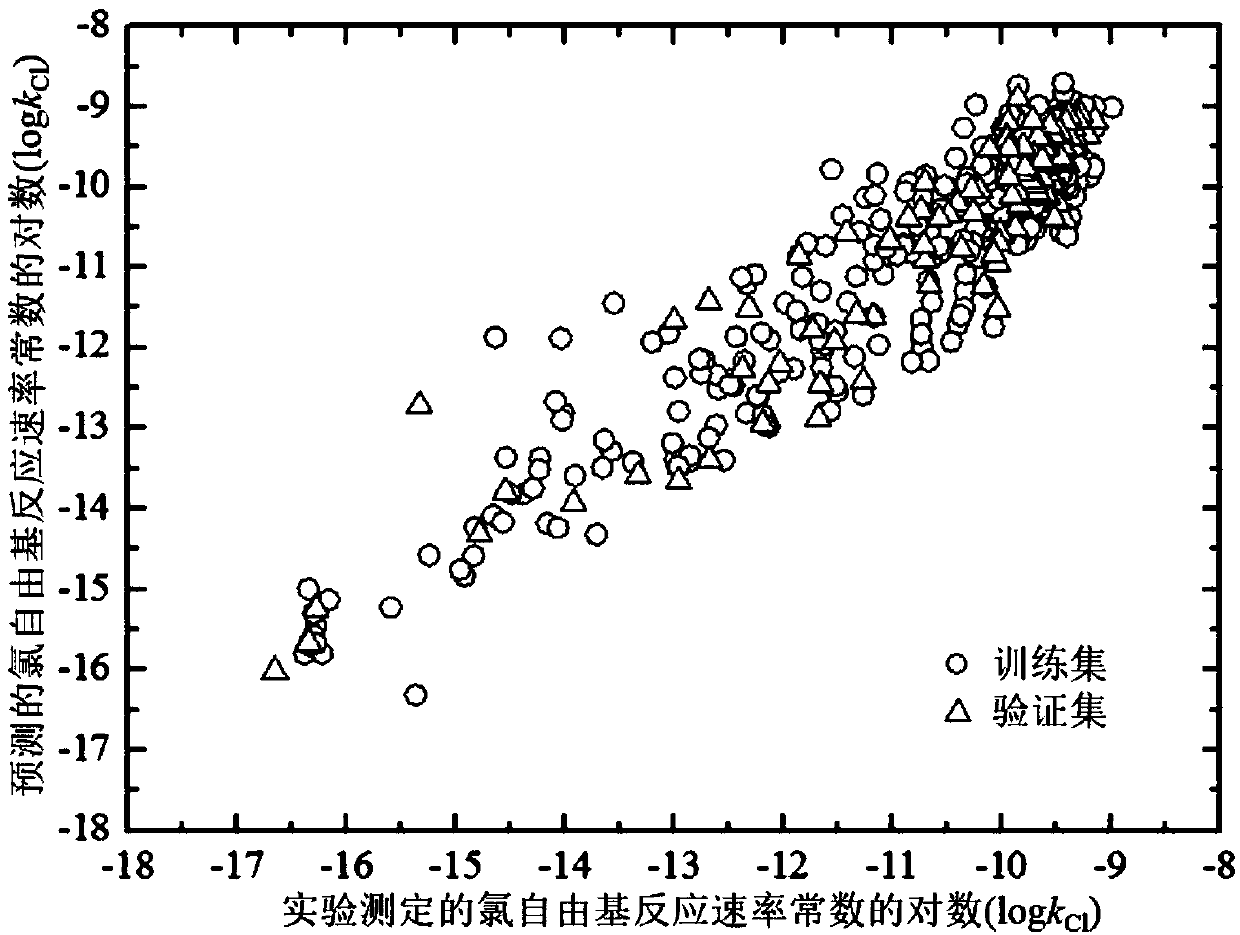
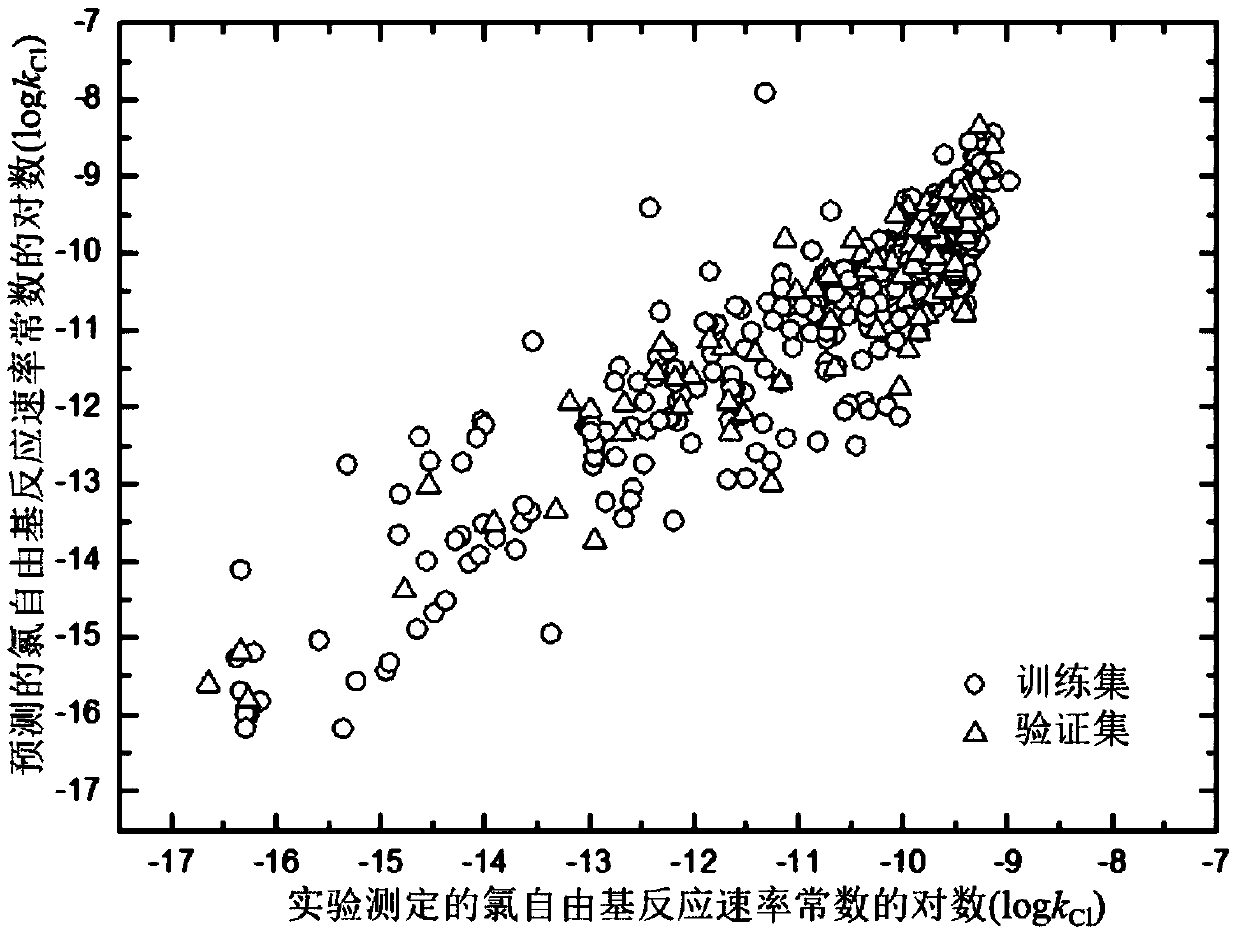
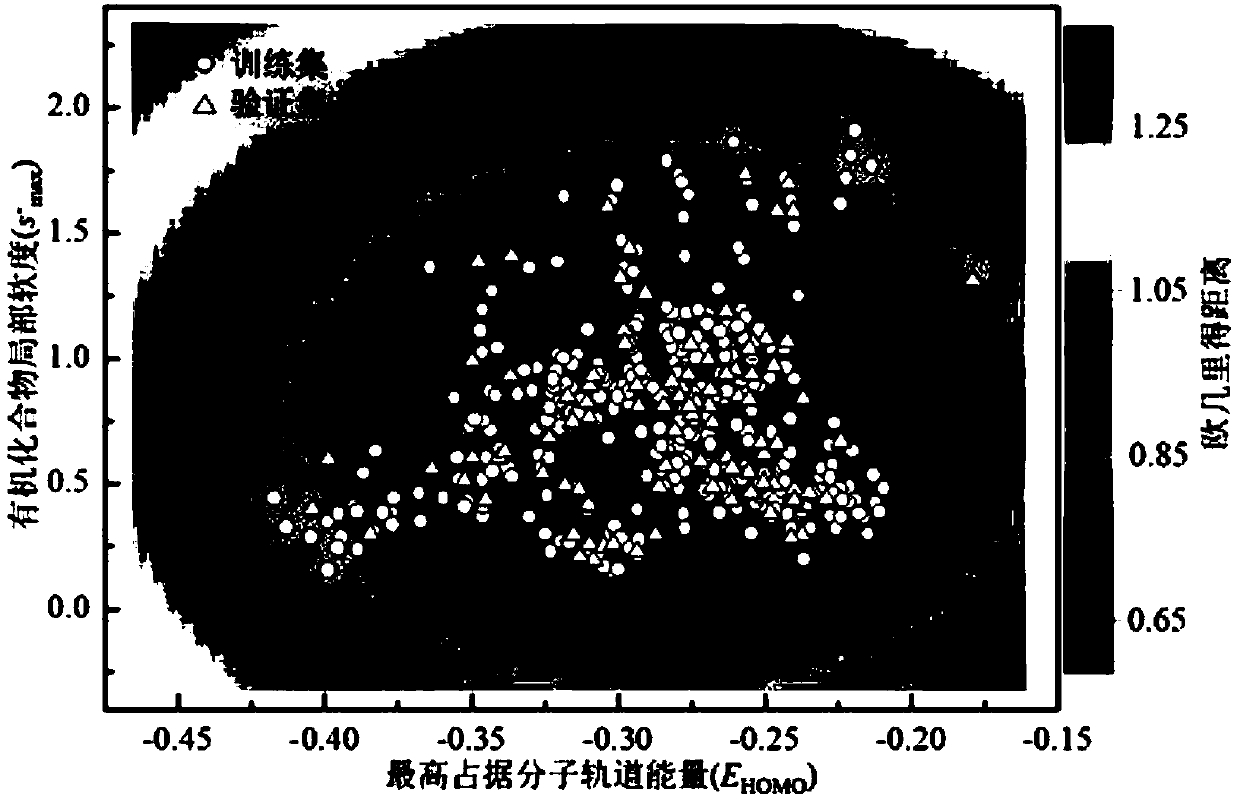
Method for adopting quantitative structure-activity relation model to predict reaction rate constant of chlorine free radical of organic chemicals
A technology of reaction rate constants and organic chemicals, applied in electrical digital data processing, special data processing applications, instruments, etc., can solve problems such as poor interpretation of model mechanism, narrow application domain, and neglect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for predicting the reaction rate constant of chlorine radicals of organic chemicals, comprising the steps of:
[0051] First, data collection: the room temperature (298K) k of organic compounds is collected from a large number of literature and books Cl value, if an organic compound contains k from more than one source Cl value, take the average value. Finally, the k of 506 organic compounds with a temperature of 298K are obtained Cl Value, organic compounds are alkanes, alkenes, alkynes, organic alcohols, ethers, ketones, aldehydes, acids, esters, thio organic compounds, acyl organic compounds, organic amines, silicones, polycyclic aromatic hydrocarbons and their substitutes, heterocyclic compounds And its derivatives, nitro substitutes or halogenated organic compounds.
[0052] Secondly, electronic structure optimization and data set grouping: In the quantum chemical calculation software Gaussian09, the structures of all organic chemicals in the data set w...
Embodiment 2
[0063] Given an organic compound 6-nitro-m-cresol, to predict its logk Cl value. First, based on the structural information of 6-nitro-m-cresol, the electronic structure was optimized using the Gaussian09 software package. E is then calculated based on the above results HOMO ,#X:C,qH ave , qC ave , (E LUMO -E HOMO ) 2 , #nonHatom: C, S, s - max , #H values are -0.261,0,0.168,-0.091,0.022,1.571,3.412,0.562,7 respectively. Calculation of d for organic chemicals using AMBIT Discovery software i The value is 0.486 (Cl The predicted value is -10.716, the experimental value is -10.712, and the residual value is 0.0037. The predicted value is in good agreement with the experimental value.
Embodiment 3
[0065] Given an organic compound crotonaldehyde, to predict its logk Cl value. First, based on the structural information of crotonaldehyde, the electronic structure was optimized using the Gaussian09 software package. E is then calculated based on the above results HOMO ,#X:C,qH ave , qC ave , (E LUMO -E HOMO ) 2 , #nonHatom: C, S, s - max , #H values are -0.264, 0, 0.142, -0.139, 0.038, 1.25, 2.743, 1.184, 6 respectively. Calculation of d for organic chemicals using AMBIT Discovery software i The value is 0.388 (Cl The predicted value is -9.753, the experimental value is -9.569, and the residual value is 0.1837. The predicted value is in good agreement with the experimental value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com